Abstract
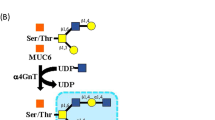
Gastric-type adenocarcinoma (GA) is an aggressive subtype of cancer of the uterine cervix. Several immunohistochemical markers for gastric mucins, such as mucin 6 (MUC6) and N-acetylglucosamine α1 → 4galactose → R (αGlcNAc-R), which is recognized by HIK1083 antibody, have been introduced for diagnosis of GA and lobular endocervical glandular hyperplasia (LEGH). However, MUC6 is also expressed in normal endocervical glands and HIK1083 antibody has limited availability. Trefoil factor family 2 protein (TFF2) is secreted by gastric, but not normal endocervical glands. Here, we evaluated TFF2 immunostaining for detection of a gastric immunophenotype in endocervical glandular lesions. We compared TFF2, αGlcNAc-R, and MUC6 expression in 103 endocervical glandular lesions: LEGH (n = 23), adenocarcinoma in situ/microinvasive adenocarcinoma (AIS–MIA) (n = 29), and invasive adenocarcinoma (usual type [UA], n = 26; GA, n = 11; intestinal type [IA], n = 2; signet ring cell type [Sig], n = 2; and mucinous adenocarcinoma not otherwise specified [NOS], n = 10). TFF2 and αGlcNAc-R expression was completely concordant in each subtype: LEGH (100%), AIS–MIA (44.8%), UA (26.9%), GA (90.9%), IA (100%), Sig (0%), and NOS (20%). TFF2 staining scores were significantly correlated with those of αGlcNAc-R in these lesions. TFF2 and αGlcNAc-R immunoreactivity was present in cytoplasmic mucins and luminal secretions. TFF2 and αGlcNAc-R were not expressed in the normal endocervical glands. MUC6 was frequently expressed in normal endocervical glands and endocervical glandular lesions. Endocervical adenocarcinomas sometimes stained only for MUC6. TFF2 is a promising immunohistochemical marker and its identification in uterine cervical secretion is a potentially useful diagnostic test for endocervical glandular lesions with gastric differentiation.



Similar content being viewed by others
References
Smith HO, Tiffany MF, Qualls CR, Key CR (2000) The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States - a 24-year population-based study. Gynecol Oncol 78(2):97–105. https://doi.org/10.1006/gyno.2000.5826
Wilbur DC, Colgan TJ, Ferenczy AS, Hirchowiz L, Loening T, McCluggage WG, Mikami Y, Park KJ, Ronnett BM, Schneider A, Soslow R, Wells M, Wright T (2014) Glandular tumours and precursors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH (eds) WHO classification of tumours of female reproductive organs. Internat. Agency for Research on Cancer, Lyon, pp 183–189
Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, Nishimura R (2007) Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 31(5):664–672. https://doi.org/10.1097/01.pas.0000213434.91868.b0
Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, Yamaguchi S, Nishimura R (2010) Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol 177(5):2169–2175. https://doi.org/10.2353/ajpath.2010.100323
Karamurzin YS, Kiyokawa T, Parkash V, Jotwani AR, Patel P, Pike MC, Soslow RA, Park KJ (2015) Gastric-type endocervical adenocarcinoma. An aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol 39(11):1449–1457. https://doi.org/10.1097/PAS.0000000000000532
Ishii K, Hosaka N, Toki T, Momose M, Hidaka E, Tsuchiya S, Katsuyama T (1998) A new view of the so-called adenoma malignum of the uterine cervix. Virchows Arch 432(4):315–322
Nucci MR, Clement PB, Young RH (1999) Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol 23(8):886–891. https://doi.org/10.1016/j.ygyno.2011.05.006
Mikami Y, Kyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, Manabe T, Akahira JI, Ito K, Tase T, Yaegashi N, Sato I, Tateno H, Naganuma H (2004) Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and ‘adenoma malignum’. Mod Pathol 17(8):962–972. https://doi.org/10.1038/modpathol.3800148
Kondo T, Hashi A, Murata S, Nakazawa T, Yuminamochi T, Nara M, Hoshi K, Katoh R (2005) Endocervical adenocarcinomas associated with lobular endocervical glandular hyperplasia: a report of four cases with histochemical and immunohistochemical analyses. Mod Pathol 18(9):1199–1210. https://doi.org/10.1038/modpathol.3800403
Nara M, Hashi A, Murata S-I, Kondo T, Yuminamochi T, Nakazawa K, Katoh R, Hoshi K (2007) Lobular endocervical glandular hyperplasia as a presumed precursor of cervical adenocarcinoma independent of human papillomavirus infection. Gynecol Oncol 106(2):289–298. https://doi.org/10.1016/j.ygyno.2007.03.044
Kawauchi S, Kusuda T, Liu XP, Suehiro Y, Kaku T, Mikami Y, Takeshita M, Nakao M, Chochi Y, Sasaki K (2008) Is lobular endocervical glandular hyperplasia a cancerous precursor of minimal deviation adenocarcinoma? A comparative molecular-genetic and immunohistochemical study. Am J Surg Pathol 32(12):1807–1815. https://doi.org/10.1097/PAS.0b013e3181883722
Liao SY, Rodgers WH, Kauderer J, Darcy KM, Carter R, Susumu N, Nagao S, Walker JL, Hatae M, Stanbridge EJ (2013) Endocervical glandular neoplasia associated with lobular endocervical glandular hyperplasia is HPV-independent and correlates with carbonic anhydrase-IX expression: a Gynaecological Oncology Group Study. Br J Cancer 108(3):613–620. https://doi.org/10.1038/bjc.2012.578
Mikami Y, Minamiguchi S, Teramoto N, Nagura M, Haga H, Konishi I (2013) Carbonic anhydrase type IX expression in lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Pathol Res Pract 209(3):173–178. https://doi.org/10.1016/j.prp.2012.12.003
Talia KL, Stewart CJ, Howitt BE, Nucci MR, McCluggage WG (2017) HPV-negative gastric type adenocarcinoma in situ of the cervix: a spectrum of rare lesions exhibiting gastric and intestinal differentiation. Am J Surg Pathol 41(8):1023–1033. https://doi.org/10.1097/PAS.0000000000000855
Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K (1996) Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318:409–416
Utsugi K, Hirai Y, Takeshima N, Akiyama F, Sakurai S, Hasumi K (1999) Utility of the monoclonal antibody HIK1083 in the diagnosis of adenoma malignum of the uterine cervix. Gynecol Oncol 75(3):345–348. https://doi.org/10.1006/gyno.1999.5622
Ota H, Harada O, Uehara T, Hayama M, Ishii K (2011) Aberrant expression of TFF1, TFF2, and PDX1 and their diagnostic value in lobular endocervical glandular hyperplasia. Am J Clin Pathol 135(2):253–261. https://doi.org/10.1309/ajcpqmao3pw4ogof
Yamanoi K, Ishii K, Tsukamoto M, Asaka S, Nakayama J (2018) Gastric gland mucin-specific O-glycan expression decreases as tumor cells progress from lobular endocervical gland hyperplasia to cervical mucinous carcinoma, gastric type. Virchows Arch 473(3):305–311. https://doi.org/10.1007/s00428-018-2381-6
Maeda D (2015) Utility of claudin-18 and p16 immunohistochemistry for distinguishing gastric-type adenocarcinoma from other subtypes of cervical adenocarcinoma. Mod Pathol 28:296A–297A
Maeda D (2016) Expression of claudin-18, a novel gastric marker, is associated with poor prognosis in cervical adenocarcinoma. Mod Pathol 29:296A–296A
Pastorekova S, Parkkila S, Parkkila AK, Opavsky R, Zelnik V, Saarnio J, Pastorek J (1997) Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 112(2):398–408
Tafreshi NK, Lloyd MC, Bui MM, Gillies RJ, Morse DL (2014) Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcell Biochem 75:221–254. https://doi.org/10.1007/978-94-007-7359-2_12
Kende AI, Carr NJ, Sobin LH (2003) Expression of cytokeratins 7 and 20 in carcinomas of the gastrointestinal tract. Histopathology 42(2):137–140
Weikel W, Wagner R, Moll R (1987) Characterization of subcolumnar reserve cells and other epithelia of human uterine cervix. Demonstration of diverse cytokeratin polypeptides in reserve cells. Virchows Arch B Cell Pathol Incl Mol Pathol 54(2):98–110
Ota H, Hayama M, Nakayama J, Hidaka H, Honda T, Ishii K, Fukushima M, Uehara T, Kurihara M, Ishihara K, Hotta K, Katsuyama T (2001) Cell lineage specificity of newly raised monoclonal antibodies against gastric mucins in normal, metaplastic, and neoplastic human tissues and their application to pathology diagnosis. Am J Clin Pathol 115(1):69–79. https://doi.org/10.1309/AMUR-K5L3-M2DN-2DK5
Nakajima K, Ota H, Zhang MX, Sano K, Honda T, Ishii K, Nakayama J (2003) Expression of gastric gland mucous cell-type mucin in normal and neoplastic human tissues. J Histochem Cytochem 51(12):1689–1698. https://doi.org/10.1177/002215540305101213
Zhang MX, Nakayama J, Hidaka E, Kubota S, Yan J, Ota H, Fukuda M (2001) Immunohistochemical demonstration of alpha1,4-N-acetylglucosaminyltransferase that forms GlcNAcalpha1,4Galbeta residues in human gastrointestinal mucosa. J Histochem Cytochem 49(5):587–596. https://doi.org/10.1177/002215540104900505
Bartman AE, Buisine MP, Aubert JP, Niehans GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE, Ho SB (1998) The MUC6 secretory mucin gene ts expressed in a wide variety of epithelial tissues. J Pathol 186(4):398–405. https://doi.org/10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X
Hoffmann W, Jagla W, Wiede A (2001) Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol 16(1):319–334. https://doi.org/10.14670/HH-16.319
Hanisch FG, Bonar D, Schloerer N, Schroten H (2014) Human trefoil factor 2 is a lectin that binds alpha-GlcNAc-capped mucin glycans with antibiotic activity against helicobacter pylori. J Biol Chem 289(40):27363–27375. https://doi.org/10.1074/jbc.M114.597757
Hoffmann W (2015) TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int J Oncol 47(3):806–816. https://doi.org/10.3892/ijo.2015.3090
Oncology FCG (2014) FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet 125(2):97–98. https://doi.org/10.1016/j.ijgo.2014.02.003
Yuan CT, Lin MC, Kuo KT, Wang TH, Mao TL (2016) Gastric-type adenocarcinoma in situ of uterine cervix: cytological and histopathological features of two cases. Virchows Arch 469(3):351–356. https://doi.org/10.1007/s00428-016-1978-x
Halimi SA, Maeda D, Shinozaki-Ushiku A, Koso T, Matsusaka K, Tanaka M, Arimoto T, Oda K, Kawana K, Yano T, Fujii T, Fukayama M (2013) Claudin-18 overexpression in intestinal-type mucinous borderline tumour of the ovary. Histopathology 63(4):534–544. https://doi.org/10.1111/his.12182
Kushima R, Hattori T (1993) Histogenesis and characteristics of gastric-type adenocarcinomas in the stomach. J Cancer Res Clin Oncol 120(1–2):103–111
Fujimori Y, Akamatsu T, Ota H, Katsuyama T (1995) Proliferative markers in gastric carcinoma and organoid differentiation. Hum Pathol 26(7):725–734
Kobayashi M, Fujinaga Y, Ota H (2014) Reappraisal of the Immunophenotype of pancreatic intraductal papillary mucinous neoplasms (IPMNs)-gastric pyloric and small intestinal immunophenotype expression in gastric and intestinal type IPMNs. Acta Histochem Cytochem 47(2):45–57. https://doi.org/10.1267/ahc.13027
Stolnicu S, Barsan I, Hoang L, Patel P, Chiriboga L, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Pike MC, Oliva E, Park KJ, Soslow RA (2018) Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol 42(8):989–1000. https://doi.org/10.1097/PAS.0000000000001090
Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA (1993) Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology 105(4):1110–1116
Acknowledgements
We thank Kayo Suzuki and Misako Yamada, research center for supports to advanced science, Shinshu University for providing expert technical assistance, and Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was funded by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) for H.O. (26460673).
Author information
Authors and Affiliations
Contributions
Shiho Asaka: acquisition of data; analysis and interpretation of data; drafting of the manuscript; immunohistochemistry staining. Tomoyuki Nakajima: acquisition of data; analysis and interpretation of data; immunohistochemistry staining. Masanobu Momose: acquisition of data; analysis and interpretation of data; immunohistochemistry staining. Tsutomu Miyamoto: material support; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Takeshi Uehara: material support; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Hiroyoshi Ota: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtaining funding; administrative, technical, or study supervision. All authors contributed to discussions and gave their final approval for the submitted manuscript.
Corresponding author
Ethics declarations
This study was reviewed and approved by the medical ethics committee of the Shinshu University School of Medicine, Japan (project no. 1875, approved on 6 December, 2011).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Asaka, S., Nakajima, T., Momose, M. et al. Trefoil factor family 2 protein: a potential immunohistochemical marker for aiding diagnosis of lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Virchows Arch 474, 79–86 (2019). https://doi.org/10.1007/s00428-018-2469-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2469-z