Abstract
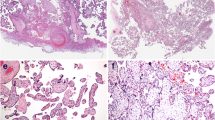
The placental tissues of pregnancy-induced hypertension (PIH) patients exhibit multiple infarctions, acute atherosis, distal villous hypoplasia, and increased syncytial knots. However, these findings are not observed in all cases of PIH; thus, the significance of these changes in PIH is still unclear. We studied the frequency of histopathological changes of placental tissue in the subgroups of PIH, such as mild and severe PIH and early-onset (< 34 weeks) and late-onset (≥ 34 weeks) PIH. One hundred seven cases of PIH diagnosed at the Shinshu University Hospital, Matsumoto, Japan, between 2008 and 2014 were collected. PIH includes preeclampsia and gestational hypertension. The pathologic changes evaluated in the placenta were multiple infarctions, acute atherosis, distal villous hypoplasia, and increased syncytial knots. Placental tissues of patients with early-onset PIH demonstrated acute atherosis resulting from the incomplete remodeling of the spiral arteries and distal villous hypoplasia and increased syncytial knots reflecting placental hypoxia/ischemia much more frequently than those with late-onset PIH (all p < 0.001). The frequencies of multiple infarctions did not show a statistical difference between early-onset PIH and late-onset PIH. Moreover, there were no significant differences in the frequencies of histopathological features of placental tissue between mild PIH and severe PIH. Early-onset PIH exhibited histopathological changes of placental tissue consistent with the two-stage disorder theory more frequently than late-onset PIH. These findings support the idea that early-onset PIH and late-onset PIH are distinct entities or different extremes of the PIH spectrum.

Similar content being viewed by others
References
Duley L (2009) The global impact of pre-eclampsia and eclampsia. Semin Perinatol 33(3):130–137. https://doi.org/10.1053/j.semperi.2009.02.010
WHO Guidelines Approved by the Guidelines Review Committee (2011) WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. World Health Organization, Geneve
Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy (2013) Obstet Gynecol 122(5):1122–1131. https://doi.org/10.1097/01.aog.0000437382.03963.88
Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y (2013) Outline of definition and classification of “pregnancy induced hypertension (PIH)”. Hypertens Res Pregnancy 1(1):3–4. https://doi.org/10.14390/jsshp.1.3
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R (2010) Pre-eclampsia. Lancet 376(9741):631–644. https://doi.org/10.1016/S0140-6736(10)60279-6
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728):1592–1594. https://doi.org/10.1126/science.1111726
Roberts JM, Hubel CA (2009) The two stage model of preeclampsia: variations on the theme. Placenta 30(Suppl A):S32–S37. https://doi.org/10.1016/j.placenta.2008.11.009
Pijnenborg R, Anthony J, Davey DA, Ress A, Tiltman A, Vercruysse L, van Assche A (1991) Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98(7):648–655. https://doi.org/10.1111/j.1471-0528.1991.tb13450.x
Prefumo F, Sebire NJ, Thilaganathan B (2004) Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod 19(1):206–209. https://doi.org/10.1093/humrep/deh037
Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH (2001) Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol 18(5):441–449. https://doi.org/10.1046/j.0960-7692.2001.00572.x
Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K (2009) First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat Diagn 29(8):781–789. https://doi.org/10.1002/pd.2287
Burger O, Pick E, Zwickel J, Klayman M, Meiri H, Slotky R, Mandel S, Rabinovitch L, Paltieli Y, Admon A, Gonen R (2004) Placental protein 13 (PP-13): effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta 25(7):608–622. https://doi.org/10.1016/j.placenta.2003.12.009
Saxena AR, Seely EW, Rich-Edwards JW, Wilkins-Haug LE, Karumanchi SA, McElrath TF (2013) First trimester PAPP-A levels correlate with sFlt-1 levels longitudinally in pregnant women with and without preeclampsia. BMC Pregnancy Childbirth 13:85. https://doi.org/10.1186/1471-2393-13-85
Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM (2002) Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 87(4):1762–1767. https://doi.org/10.1210/jcem.87.4.8430
Allen RE, Rogozinska E, Cleverly K, Aquilina J, Thangaratinam S (2014) Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 182:194–201. https://doi.org/10.1016/j.ejogrb.2014.09.027
De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D'Anna R (2008) Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand 87(8):837–842. https://doi.org/10.1080/00016340802253759
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111(5):649–658. https://doi.org/10.1172/jci17189
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group (2006) Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355(10):992–1005. https://doi.org/10.1056/NEJMoa055352
Speer PD, Powers RW, Frank MP, Harger G, Markovic N, Roberts JM (2008) Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with small-for-gestational-age infants. Am J Obstet Gynecol 198(1):112.e1–112.e7. https://doi.org/10.1016/j.ajog.2007.05.052
Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM (2004) Placental pathology. American Registry of Pathology, Armed Forces Institute of Pathology, Washington, DC
Benirschke K, Graham B, Baergen RN (2012) Pathology of the human placenta, 6th edn. Springer, Verlin. https://doi.org/10.1007/978-3-642-23941-0
Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, Prashner HR, Waters BL, for the Society for Pediatric Pathology, Perinatal Section, Maternal Vascular Underperfusion Nosology Committee (2004) Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 7(3):237–249. https://doi.org/10.1007/s10024-003-8083-2
Faye-Petersen OM, Heller DS, Joshi VV (2006) Handbook of placental pathology, 2nd edn. Taylor & Francis, Oxfordshire
Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B (2003) The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol 189(4):1173–1177. https://doi.org/10.1067/S0002-9378(03)00576-3
Zhang P, Schmidt M, Cook L (2006) Maternal vasculopathy and histologic diagnosis of preeclampsia: poor correlation of histologic changes and clinical manifestation. Am J Obstet Gynecol 194(4):1050–1056. https://doi.org/10.1016/j.ajog.2005.10.196
Corrêa RR, Gilio DB, Cavellani CL et al (2008) Placental morphometrical and histopathology changes in the different clinical presentations of hypertensive syndromes in pregnancy. Arch Gynecol Obstet 277(3):201–206. https://doi.org/10.1007/s00404-007-0452-z
Stark MW, Clark L, Craver RD (2014) Histologic differences in placentas of preeclamptic/eclamptic gestations by birthweight, placental weight, and time of onset. Pediatr Dev Pathol 17(3):181–189. https://doi.org/10.2350/13-09-1378-oa.1
Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AEP, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PGJ, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ (2016) Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med 140(7):698–713. https://doi.org/10.5858/arpa.2015-0225-CC
Valensise H, Bezzeccheri V, Rizzo G, Tranquilli AL, Garzetti GG, Romanini C (1993) Doppler velocimetry of the uterine artery as a screening test for gestational hypertension. Ultrasound Obstet Gynecol 3(1):18–22. https://doi.org/10.1046/j.1469-0705.1993.03010018.x
Arakaki T, Hasegawa J, Nakamura M, Hamada S, Muramoto M, Takita H, Ichizuka K, Sekizawa A (2015) Prediction of early- and late-onset pregnancy-induced hypertension using placental volume on three-dimensional ultrasound and uterine artery Doppler. Ultrasound Obstet Gynecol 45(5):539–543. https://doi.org/10.1002/uog.14633
Pinheiro CC, Rayol P, Gozzani L, dos Reis LM, Zampieri G, Dias CB, Woronik V (2014) The relationship of angiogenic factors to maternal and neonatal manifestations of early-onset and late-onset preeclampsia. Prenat Diagn 34(11):1084–1092. https://doi.org/10.1002/pd.4432
Lisonkova S, Joseph KS (2013) Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 209(6):544.e1–544.e12. https://doi.org/10.1016/j.ajog.2013.08.019
Valensise H, Vasapollo B, Gagliardi G, Novelli GP (2008) Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 52(5):873–880. https://doi.org/10.1161/hypertensionaha.108.117358
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the medical ethics committee at the Shinshu University School of Medicine (Project #3265 was approved on November 2, 2015).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tateishi, A., Ohira, S., Yamamoto, Y. et al. Histopathological findings of pregnancy-induced hypertension: histopathology of early-onset type reflects two-stage disorder theory. Virchows Arch 472, 635–642 (2018). https://doi.org/10.1007/s00428-018-2315-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2315-3