Abstract
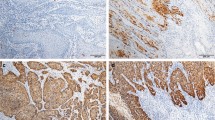
Meta-analyses show that approximately half of all squamous cell carcinomas (SCCs) of the penis are associated with a human papillomavirus (HPV) infection. As data about the tumour microenvironment of HPV-positive and HPV-negative penile carcinomas is scarce and conflicting, we examined tumour-infiltrating lymphocyte populations in such cases. The HPV status of 28 penile SCCs was determined by polymerase chain reaction, while the number and distribution of different lymphocyte populations were analysed by immunohistochemistry on whole sections of paraffin-embedded tumour specimens. The average number of tumour-infiltrating T cells in HPV-associated SCC was higher than in HPV-negative SCC, and their phenotype showed strong polarization towards a T helper 1 and cytotoxic immune response. In addition, we identified more tumour-infiltrating regulatory T cells in HPV-positive carcinomas, which might represent a mechanism of immune evasion. The present study provides further evidence that the tumour microenvironment of HPV-positive carcinomas differs from that of HPV-negative carcinomas.



Similar content being viewed by others
References
Palefsky JM (2007) HPV infection in men. Dis Markers 23(4):261–272
Pow-Sang MR, Ferreira U, Pow-Sang JM, Nardi AC, Destefano V (2010) Epidemiology and natural history of penile cancer. Urology 76(2):S2–6. doi:10.1016/j.urology.2010.03.003
Curado E, Shin, Storm, Ferlay, Heanue, Boyle (ed) (2007) Cancer Incidence in Five Continents, Vol. IX, vol 160. IARC Scientific Publications
Backes DM, Kurman RJ, Pimenta JM, Smith JS (2009) Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 20(4):449–457. doi:10.1007/s10552-008-9276-9
Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de Sanjose S (2009) Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol 62(10):870–878. doi:10.1136/jcp.2008.063149
Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, Pirog EC (2001) Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol 159(4):1211–1218. doi:10.1016/s0002-9440(10)62506-0
Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD (1995) Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst 87(22):1705–1709
Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ (2009) Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 27(2):141–150. doi:10.1007/s00345-008-0302-z
Mannweiler S, Sygulla S, Winter E, Regauer S (2013) Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol 69(1):73–81. doi:10.1016/j.jaad.2012.12.973
Senba M, Mori N, Fujita S, Jutavijittum P, Yousukh A, Toriyama K, Wada A (2010) Relationship among human papillomavirus infection, p16(INK4a), p53 and NF-kappaB activation in penile cancer from northern Thailand. Oncol Lett 1(4):599–603. doi:10.3892/ol_00000106
Gronhoj Larsen C, Gyldenlove M, Jensen DH, Therkildsen MH, Kiss K, Norrild B, Konge L, von Buchwald C (2014) Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer 110(6):1587–1594. doi:10.1038/bjc.2014.42
Schewe C, Goldmann T, Grosser M, Zink A, Schluns K, Pahl S, Ulrichs T, Kaufmann SH, Nerlich A, Baretton GB, Dietel M, Vollmer E, Petersen I (2005) Inter-laboratory validation of PCR-based detection of Mycobacterium tuberculosis in formalin-fixed, paraffin-embedded tissues. Virchows Arch 447(3):573–585. doi:10.1007/s00428-005-1233-3
Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T (2006) Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 44(2):504–512. doi:10.1128/jcm. 44.2.504-512.2006
Schmitt M, Dondog B, Waterboer T, Pawlita M (2008) Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J Clin Microbiol 46(3):1050–1059. doi:10.1128/jcm. 02227-07
Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD (1998) Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 338(7):423–428. doi:10.1056/nejm199802123380703
Nicholls PK, Klaunberg BA, Moore RA, Santos EB, Parry NR, Gough GW, Stanley MA (1999) Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology 265(2):365–374. doi:10.1006/viro.1999.0060
Wilgenburg BJ, Budgeon LR, Lang CM, Griffith JW, Christensen ND (2005) Characterization of immune responses during regression of rabbit oral papillomavirus infections. Comp Med 55(5):431–439
Kovacic MB, Katki HA, Kreimer AR, Sherman ME (2008) Epidemiologic analysis of histologic cervical inflammation: relationship to human papillomavirus infections. Hum Pathol 39(7):1088–1095. doi:10.1016/j.humpath.2007.12.002
Loddenkemper C, Hoffmann C, Stanke J, Nagorsen D, Baron U, Olek S, Huehn J, Ritz JP, Stein H, Kaufmann AM, Schneider A, Cichon G (2009) Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci 100(6):1112–1117
Kojima S, Kawana K, Fujii T, Yokoyama T, Miura S, Tomio K, Tomio A, Yamashita A, Adachi K, Sato H, Nagamatsu T, Schust DJ, Kozuma S, Taketani Y (2011) Characterization of gut-derived intraepithelial lymphocyte (IEL) residing in human papillomavirus (HPV)-infected intraepithelial neoplastic lesions. Am J Reprod Immunol (New York, NY: 1989) 66(5):435–443. doi:10.1111/j.1600-0897.2011.01041.x
Hibma MH (2012) The immune response to papillomavirus during infection persistence and regression. Open Virol J 6:241–248. doi:10.2174/1874357901206010241
Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, Kos F, Teague J, Jiang Y, Barat NC, Jungbluth AA (2010) Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol 185(11):7107–7114. doi:10.4049/jimmunol.1002756
Monnier-Benoit S, Mauny F, Riethmuller D, Guerrini JS, Capilna M, Felix S, Seilles E, Mougin C, Pretet JL (2006) Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol Oncol 102(1):22–31. doi:10.1016/j.ygyno.2005.11.039
Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, Chepeha D, Kumar B, Cordell K, Eisbruch A, Taylor J, Moyer J, Bradford C, D’Silva N, Carey T, McHugh J, Wolf G (2012) Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope 122(1):121–127. doi:10.1002/lary.22133
Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT (2009) The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 74(2):553–561. doi:10.1016/j.ijrobp.2009.02.015
Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V, Job S, de Reynies A, Noel G, Wasylyk B, Clavel C, Abecassis J (2013) CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer J Int du Cancer 132(2):E26–36. doi:10.1002/ijc.27776
Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T (2012) Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 7(6):e38711. doi:10.1371/journal.pone.0038711
Cubilla AL, Lloveras B, Alejo M, Clavero O, Chaux A, Kasamatsu E, Monfulleda N, Tous S, Alemany L, Klaustermeier J, Munoz N, Quint W, de Sanjose S, Bosch FX (2011) Value of p16(INK)(4)(a) in the pathology of invasive penile squamous cell carcinomas: a report of 202 cases. Am J Surg Pathol 35(2):253–261. doi:10.1097/PAS.0b013e318203cdba
Ferrandiz-Pulido C, Masferrer E, de Torres I, Lloveras B, Hernandez-Losa J, Mojal S, Salvador C, Morote J, Ramon Cajal S, Pujol RM, Garcia-Patos V, Toll A (2013) Identification and genotyping of human papillomavirus in a Spanish cohort of penile squamous cell carcinomas: correlation with pathologic subtypes, p16(INK4a) expression, and prognosis. J Am Acad Dermatol 68(1):73–82. doi:10.1016/j.jaad.2012.05.029
Cubilla AL, Lloveras B, Alejo M, Clavero O, Chaux A, Kasamatsu E, Velazquez EF, Lezcano C, Monfulleda N, Tous S, Alemany L, Klaustermeier J, Munoz N, Quint W, de Sanjose S, Bosch FX (2010) The basaloid cell is the best tissue marker for human papillomavirus in invasive penile squamous cell carcinoma: a study of 202 cases from Paraguay. Am J Surg Pathol 34(1):104–114. doi:10.1097/PAS.0b013e3181c76a49
Krustrup D, Jensen HL, van den Brule AJ, Frisch M (2009) Histological characteristics of human papilloma-virus-positive and -negative invasive and in situ squamous cell tumours of the penis. Int J Exp Pathol 90(2):182–189. doi:10.1111/j.1365-2613.2008.00636.x
Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP (2003) CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421(6925):852–856. doi:10.1038/nature01441
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. doi:10.1038/nrc3245
Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, Smit VT, Langeveld AP, Jansen JC, van der Burg SH (2012) Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer J Int du Cancer 131(2):E74–85. doi:10.1002/ijc.26497
Albers A, Abe K, Hunt J, Wang J, Lopez-Albaitero A, Schaefer C, Gooding W, Whiteside TL, Ferrone S, DeLeo A, Ferris RL (2005) Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res 65(23):11146–11155. doi:10.1158/0008-5472.can-05-0772
Hoffmann TK, Arsov C, Schirlau K, Bas M, Friebe-Hoffmann U, Klussmann JP, Scheckenbach K, Balz V, Bier H, Whiteside TL (2006) T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int J Cancer J Int du Cancer 118(8):1984–1991. doi:10.1002/ijc.21565
Bourgault Villada I, Moyal Barracco M, Ziol M, Chaboissier A, Barget N, Berville S, Paniel B, Jullian E, Clerici T, Maillere B, Guillet JG (2004) Spontaneous regression of grade 3 vulvar intraepithelial neoplasia associated with human papillomavirus-16-specific CD4(+) and CD8(+) T-cell responses. Cancer Res 64(23):8761–8766. doi:10.1158/0008-5472.can-04-2455
Woo YL, van den Hende M, Sterling JC, Coleman N, Crawford RA, Kwappenberg KM, Stanley MA, van der Burg SH (2010) A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer J Int du Cancer 126(1):133–141. doi:10.1002/ijc.24804
Seresini S, Origoni M, Caputo L, Lillo F, Longhi R, Vantini S, Paganoni AM, Protti MP (2010) CD4+ T cells against human papillomavirus-18 E7 in patients with high-grade cervical lesions associate with the absence of the virus in the cervix. Immunology 131(1):89–98. doi:10.1111/j.1365-2567.2010.03277.x
Dillon S, Sasagawa T, Crawford A, Prestidge J, Inder MK, Jerram J, Mercer AA, Hibma M (2007) Resolution of cervical dysplasia is associated with T-cell proliferative responses to human papillomavirus type 16 E2. J Gen Virol 88(Pt 3):803–813. doi:10.1099/vir. 0.82678-0
Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH (2009) Preclinical models of HPV+ and HPV- HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck 31(7):911–918. doi:10.1002/hed.21040
Whiteside TL (2012) What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol 22(4):327–334. doi:10.1016/j.semcancer.2012.03.004
Whiteside TL, Schuler P, Schilling B (2012) Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther 12(10):1383–1397. doi:10.1517/14712598.2012.707184
van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R (2007) Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A 104(29):12087–12092. doi:10.1073/pnas.0704672104
Le Poole C, Denman CJ, Arbiser JL (2008) Immunosuppression may be present within condyloma acuminata. J Am Acad Dermatol 59(6):967–974. doi:10.1016/j.jaad.2008.08.011
Cao Y, Zhao J, Lei Z, Shen S, Liu C, Li D, Liu J, Shen GX, Zhang GM, Feng ZH, Huang B (2008) Local accumulation of FOXP3+ regulatory T cells: evidence for an immune evasion mechanism in patients with large condylomata acuminata. J Immunol 180(11):7681–7686
Wansom D, Light E, Worden F, Prince M, Urba S, Chepeha DB, Cordell K, Eisbruch A, Taylor J, D’Silva N, Moyer J, Bradford CR, Kurnit D, Kumar B, Carey TE, Wolf GT (2010) Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol–Head Neck Surg 136(12):1267–1273. doi:10.1001/archoto.2010.211
Acknowledgments
The authors would like to thank F. Gocht, V. Arnemann and U. Schiller for their excellent technical assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lohneis, P., Boral, S., Kaufmann, A.M. et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch 466, 323–331 (2015). https://doi.org/10.1007/s00428-014-1713-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1713-4