Abstract
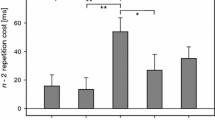
Inhibition in task switching is inferred from slower reaction times returning to a recently performed task after one intervening trial (i.e. an ABA sequence) compared to returning to a task not recently performed (CBA sequence). These n−2 repetition costs are thought to reflect the persisting inhibition of a task after its disengagement. As such, the n−2 repetition cost is an attractive tool for the researcher interested in inhibitory functioning in clinical/neurological/neuroscience disciplines. In the literature, an absence of this cost is often interpreted as an absence of inhibition, an assumption with strong implications for researchers. The current paper argues that this is not necessarily an accurate interpretation, as an absence of inhibition should lead to an n−2 repetition benefit as a task’s activation level will prime performance. This argument is supported by three instances of a computational cognitive model varying the degree of inhibition present. An inhibition model fits human n−2 repetition costs well. Removal of the inhibition—the activation-only model—predicts an n−2 repetition benefit. For the model to produce a null n−2 repetition cost, small amounts of inhibition were required—the reduced-inhibition model. The authors also demonstrate that a lateral-inhibition locus of the n−2 repetition cost cannot account for observed human data. The authors conclude that a null n−2 repetition cost provides no evidence on its own for an absence of inhibition, and propose reporting of a significant n−2 repetition benefit to be the best evidence for a lack of inhibition. Implications for theories on task switching are discussed.




Similar content being viewed by others
Notes
There are some non-inhibitory accounts of n–2 repetition costs in the literature, but to date these have been unable to explain extant data (Mayr, 2007).
Of course, one cannot be certain of no inhibition even in the presence of an n–2 repetition benefit without explicit modelling of the latent processes.
References
Altmann, E. M. (2007). Cue-independent task-specific representations in task switching: Evidence from backward inhibition. J Exp Psychol Learn Mem Cogn, 33, 892–899.
Altmann, E. M., & Gray, W. D. (2008). An integrated model of cognitive control in task switching. Psychol Rev, 115, 602–639.
Anderson, J. R. (2007). How can the human mind occur in the physical universe?. New York: Oxford University Press.
Arbuthnott, K. D. (1995). Inhibitory mechanisms in cognition: Phenomena and models. Cahiers de Psychologie Cognitive/Current Psychology of Cognition, 14, 3–45.
Arbuthnott, K. D. (2005). The influence of cue type on backward inhibition. J Exp Psychol Learn Mem Cogn, 31, 1030–1060.
Arbuthnott, K. D. (2008a). Asymmetric switch cost and backward inhibition: Carryover of activation and inhibition in switching between tasks of unequal difficulty. Can J Exp Psychol, 62, 91–100.
Arbuthnott, K. D. (2008b). The effect of task location and task type on backward inhibition. Mem Cogn, 36, 534–543.
Arbuthnott, K. D. (2009). The representational locus of spatial influence on backward inhibition. Mem Cogn, 37, 522–528.
Arbuthnott, K. D., & Campbell, J. I. D. (2003). The locus of self-inhibition in sequential retrieval. Eur J Cogn Psychol, 15, 177–194.
Arbuthnott, K. D., & Frank, J. (2000). Executive control in set switching: Residual switch costs and task-set inhibition. Can J Exp Psychol, 54, 33–41.
Arbuthnott, K. D., & Woodward, T. S. (2002). The influence of cue-task association and location on switch cost and alternating-switch cost. Can J Exp Psychol, 56, 18–29.
Blakemore, C., & Tobin, E. A. (1972). Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp Brain Res, 15(4), 439–440.
Campbell, J. I. D., & Arbuthnott, K. D. (1996). Inhibitory processing in sequential retrieval: Evidence from variable-lag repetition priming. Brain Cogn, 30, 59–80.
Druey, M. D., & Hübner, R. (2007). The role of cue-target overlap in backward inhibition under task switching. Psychon Bull Rev, 14, 749–754.
Fales, C. L., Vanek, Z. F., & Knowlton, B. J. (2006). Backward inhibition in Parkinson’s disease. Neuropsychologia, 44, 1041–1049.
Gade, M., & Koch, I. (2005). Linking inhibition to activation in the control of task sequences. Psychon Bull Rev, 12, 530–534.
Gade, M., & Koch, I. (2007). The influence of overlapping response sets on task inhibition. Mem Cogn, 35, 603–609.
Gade, M., & Koch, I. (2008). Dissociating cue-related and task-related processes in task- inhibition: Evidence from using a 2:1 cue-to-task mapping. Can J Exp Psychol, 62, 51–55.
Gilbert, S., & Shallice, T. (2001). Task switching: A PDP model. Cogn Psychol, 44, 297–337.
Gorfein, D. S., & Brown, V. R. (2007). Saying no to inhibition. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 103–124). Washington DC: American Psychological Association.
Grange, J. A., & Houghton, G. (2009). Temporal cue-target overlap is not essential for backward inhibition in task switching. Quart J Exp Psychol, 62, 2069–2080.
Grange, J. A., & Houghton, G. (2010). Heightened conflict in cue-target translation increases backward inhibition in set switching. J Exp Psychol Learn Mem Cogn, 36, 1003–1009.
Grange, J. A., & Houghton, G. (2011a). Task preparation and task inhibition: A comment on Koch, Gade, Schuch, & Philipp (2010). Psychon Bull Rev, 18, 211–216.
Grange, J.A., & Houghton, G. (2011). Inhibition in task switching: Task cues and individual differences. Paper presented at the meeting of the Experimental Psychology Society, Oxford.
Houghton, G. (1993). Inhibitory control of neurodynamics: Opponent mechanisms in sequencing and selective attention. In M. Oaksford & G. D. A. Brown (Eds.), Neurodynamics and Psychology. London: Academic Press.
Houghton, G., Pritchard, R., & Grange, J. A. (2009). The role of cue-target translation in backward inhibition of attentional set. J Exp Psychol Learn Mem Cogn, 35, 466–476.
Houghton, G., & Tipper, S. P. (1994). A model of inhibitory mechanisms in selective attention. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory mechanisms in attention, memory, and language (pp. 53–112). San Diego: Academic Press.
Hübner, R., & Druey, M. D. (2006). Response execution, selection, or activation: What is sufficient for response-related repetition effects under task shifting? Psychol Res, 70, 245–261.
Isaacson, J. S., & Scanziani, M. (2011). How inhibition shapes cortical activity. Neuron, 72, 231–243.
Juvina, I. (2011a). Cognitive control: Componential and yet emergent. Top Cogn Sci, 2, 242–246.
Juvina, I. (2011b). Neural substrates of inhibitory control: A review and critique. Revista de Psihologie, 57, 135–145.
Juvina, I., & Taatgen, N. A. (2009). A repetition-suppression account of between-trial effects in a modified Stroop-task. Acta Psychol, 131, 72–84.
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., et al. (2010). Control and interference in task switching—A review. Psychol Bull, 136, 849–874.
Koch, I., Gade, M., Schuch, S., & Philipp, A. M. (2010). The role of inhibition in task switching—A review. Psychon Bull Rev, 17, 1–14.
Lebiere, C., & Best, B.J. (2009). Balancing long-term reinforcement and short-term inhibition. In Proceedings of the 31st Annual Conference of the Cognitive Science Society. Austin: Cognitive Science Society.
MacLeod, C. M. (2007). The concept of inhibition in cognition. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 3–23). Washington DC: American Psychological Association.
MacLeod, C. M., Dodd, M. D., Sheard, E. D., Wilson, D. E., & Bibi, U. (2003). In opposition to inhibition. Psychol Learn Motiv, 43, 163–214.
Mari-Beffa, P., Estevez, M. A., & Danziger, S. (2000). Stroop interference and negative priming: Problems with inferences from null results. Psychon Bull Rev, 7, 499–503.
Mari-Beffa, P., Cooper, S., & Houghton, G. Unmixing the mixing cost: Contributions from dimensional relevance and stimulus-response suppression. J Exp Psychol Hum Percept Perform (in press).
Mayr, U. (2007). Inhibition of task sets. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 27–44). Washington DC: American Psychological Association.
Mayr, U., Diedrichsen, J., Ivry, R., & Keele, S. W. (2006). Dissociating task-set selection from task-set inhibition in the prefrontal cortex. J Cogn Neurosci, 18, 14–21.
Mayr, U., & Keele, S. W. (2000). Changing internal constraints on action: The role of backward inhibition. J Exp Psychol Gen, 129, 4–26.
Moritz, S., Hübner, M., & Kluwe, R. (2004). Task switching and backward inhibition in obsessive-compulsive disorder. J Clin Exp Neuropsychol, 26, 677–683.
Norman, D.A. & Shallice, T. (1980) Attention to action: Willed and automatic control of behaviour. Center for Human Information Processing Technical Report no. 99.
Philipp, A. M., & Koch, I. (2006). Task inhibition and task repetition in task switching. Eur J Cogn Psychol, 18, 624–639.
Schneider, D. W., & Logan, G. D. (2005). Modeling task switching without switching tasks: A short-term priming account of explicitly cued performance. J Exp Psychol Gen, 134, 343–367.
Schneider, D. W., & Verbruggen, F. (2008). Inhibition of irrelevant category-response mappings. Quart J Exp Psychol, 61, 1629–1640.
Schuch, S., & Koch, I. (2003). The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Hum Percept Perform, 29, 92–105.
Sillito, A. M. (1975). The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol, 250, 305–329.
Tipper, S. P. (2001). Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. Quart J Exp Psychol, 37A, 571–590.
Whitmer, A. J., & Banich, M. T. (2007). Inhibition versus switching deficits in different forms of rumination. Psychol Sci, 18, 546–553.
Acknowledgments
The authors are grateful to Katherine Arbuthnott, Bernhard Hommel, and an anonymous reviewer for their constructive feedback. We would like to thank Katherine Arbuthnott for suggesting that we discuss lateral inhibition in this context.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A
See Table 2
Appendix B
See Table 3
Appendix C
Implementation details of the lateral-inhibition model.
In a competitive network using LI as part of a selection mechanism, each element in the set of possible responses has inhibitory connections to all the others. The strength of this inhibitory connection, w −, is usually equal for all connections and unchanging during processing. Thus any unit u i , with activation level a i will send an inhibitory signal of magnitude \( w^{ - } \times a_{i} \) to each of the others. Conversely, that unit u i will receive a combined inhibitory signal \( I = w^{ - } \sum\nolimits_{j \ne i} {a_{j} } \) from the set of units u j , j ≠ i.
To show how lateral inhibition is predicting an n−2 repetition benefit, we simulated LI in a simplified model computed in Visual Basic within Excel. In this case, each task (of 3) is represented as a single unit, with an activation level in the range [−1, 1] and a resting level of 0. A negative activation level represents a suppressed (below baseline) state and does not propagate. All three units are connected to each other via inhibitory links of equal strength. The only excitatory input to the model is an external input I ext representing the effect of a current task cue. On any trial only one task unit receives any such input, and that input builds up gradually (over 5 discrete time slices) to reach a maximum strength of 0.8. At each time t each unit u i updates its activation level a i according to
(The activations are hard-clipped to the range [−1, 1]). In Eq. 3, \( \delta \) is a decay (or recovery) parameter, which differs depending on whether the activation a i is above or below baseline. For \( a_{i} > 0, \, \delta = 0.5 \), otherwise \( \delta = 0.95 \). The terms \( I_{i}^{\text{ext}} {\text{ and }}I_{i}^{\text{inh}} , \) respectively, represent the excitatory external input, and the (internal) lateral inhibition to unit u i . The latter is defined by
where w − (=0.1) is the inhibitory weight as discussed above, and the summation is over all units other than u i (recall also that sub-baseline activations do not propagate, i.e. they are treated as 0).
Rights and permissions
About this article
Cite this article
Grange, J.A., Juvina, I. & Houghton, G. On costs and benefits of n−2 repetitions in task switching: towards a behavioural marker of cognitive inhibition. Psychological Research 77, 211–222 (2013). https://doi.org/10.1007/s00426-012-0421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-012-0421-4