Abstract
Main conclusion
In this study, we show that aberrant pre-mRNAs from non-spliced and non-polyadenylated intron-containing transgenes are channelled to the RNA silencing pathway.
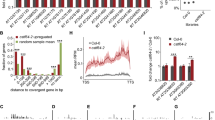
In plants, improperly processed transcripts are called aberrant RNAs (ab-RNAs) and are eliminated by either RNA silencing or RNA decay mechanisms. Ab-RNAs transcribed from intronless genes are copied by RNA-directed RNA polymerases (RDRs) into double-stranded RNAs which are subsequently cleaved by DICER-LIKE endonucleases into small RNAs (sRNAs). In contrast, ab-RNAs from intron-containing genes are suggested to be channelled post-splicing to exonucleolytic degradation. Yet, it is not clear how non-spliced aberrant pre-mRNAs are eliminated. We reasoned that transient expression of agroinfiltrated intron-containing transgenes in Nicotiana benthamiana would allow us to study the steady-state levels of non-spliced pre-mRNAs. SRNA deep sequencing of the agroinfiltrated transgenes revealed the presence of sRNAs mapping to the entire non-spliced pre-mRNA suggesting that RDRs (most likely RDR6) processed aberrant non-spliced pre-mRNAs. Primary and secondary sRNAs with lengths of 18–25 nucleotides (nt) were detected, with the most prominent sRNA size class of 22 nt. SRNAs also mapped to the terminator sequence, indicating that RDR substrates also comprised read-through transcripts devoid of polyadenylation tail. Importantly, the occurring sRNAs efficiently targeted cognate mRNA for degradation but failed to cleave the non-spliced pre-mRNA, corroborating the notion that sRNAs are not triggering RNA cleavage in the nucleus.








Similar content being viewed by others
References
Baeg K, Iwakawa HO, Tomari Y (2017) The poly(A) tail blocks RDR6 from converting self mRNAs into substrates for gene silencing. Nat Plants 3:17036
Baulcombe D (2004) RNA silencing in plants. Nature 431(7006):356–363. https://doi.org/10.1038/nature02874
Beclin C, Boutet S, Waterhouse P, Vaucheret H (2002) A branched pathway for transgene-induced RNA silencing in plants. Curr Biol 12(8):684–688
Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F Jr, Hohn T, Pooggin MM (2006) Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res 34(21):6233–6246
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science (New York, NY) 320(5880):1185–1190
Cao JY, Xu YP, Li W, Li SS, Rahman H, Cai XZ (2016) Genome-wide identification of dicer-like, argonaute, and RNA-dependent RNA polymerase gene families in brassica species and functional analyses of their arabidopsis homologs in resistance to Sclerotinia sclerotiorum. Front Plant Sci 7:1614
Chan SW, Zilberman D, Xie Z, Johansen LK, Carrington JC, Jacobsen SE (2004) RNA silencing genes control de novo DNA methylation. Science (New York, NY) 303(5662):1336
Chen HM, Chen LT, Patel K, Li YH, Baulcombe DC, Wu SH (2010) 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc Natl Acad Sci USA 107(34):15269–15274
Christie M, Carroll BJ (2011) SERRATE is required for intron suppression of RNA silencing in Arabidopsis. Plant Signal Behav 6(12):2035–2037
Christie M, Brosnan CA, Rothnagel JA, Carroll BJ (2011a) RNA decay and RNA silencing in plants: competition or collaboration? Front Plant Sci 2:99
Christie M, Croft LJ, Carroll BJ (2011b) Intron splicing suppresses RNA silencing in Arabidopsis. Plant J 68(1):159–167
Cuperus JT, Carbonell A, Fahlgren N, Garcia-Ruiz H, Burke RT, Takeda A, Sullivan CM, Gilbert SD, Montgomery TA, Carrington JC (2010) Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis. Nat Struct Mol Biol 17(8):997–1003
Dadami E, Moser M, Zwiebel M, Krczal G, Wassenegger M, Dalakouras A (2013) An endogene-resembling transgene delays the onset of silencing and limits siRNA accumulation. FEBS Lett 18(587(6)):706–710
Dadami E, Dalakouras A, Zwiebel M, Krczal G, Wassenegger M (2014) An endogene-resembling transgene is resistant to DNA methylation and systemic silencing. RNA Biol 11(7):934–941. https://doi.org/10.4161/rna.29623
Dalakouras A, Moser M, Zwiebel M, Krczal G, Hell R, Wassenegger M (2009) A hairpin RNA construct residing in an intron efficiently triggered RNA-directed DNA methylation in tobacco. Plant J 60(5):840–851. https://doi.org/10.1111/j.1365-313X.2009.04003.x
Dalakouras A, Dadami E, Bassler A, Zwiebel M, Krczal G, Wassenegger M (2015) Replicating Potato spindle tuber viroid mediates de novo methylation of an intronic viroid sequence but no cleavage of the corresponding pre-mRNA. RNA Biol 12:268–275
Dalakouras A, Wassenegger M, McMillan J, Cardoza V, Maegele I, Dadami E, Runne M, Krczal G, Wassenegger M (2016) Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front Plant Sci 7:1327. https://doi.org/10.3389/fpls.2016.01327
Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101(5):543–553
Dalmay T, Horsefield R, Braunstein TH, Baulcombe DC (2001) SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J 20(8):2069–2078
Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O (2006) Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science (New York, NY) 313(5783):68–71
Dye MJ, Gromak N, Proudfoot NJ (2006) Exon tethering in transcription by RNA polymerase II. Mol Cell 21(6):849–859
Eamens A, Wang MB, Smith NA, Waterhouse PM (2008) RNA silencing in plants: yesterday, today, and tomorrow. Plant Physiol 147(2):456–468. https://doi.org/10.1104/pp.108.117275
Elvira-Matelot E, Bardou F, Ariel F, Jauvion V, Bouteiller N, Le Masson I, Cao J, Crespi MD, Vaucheret H (2016) The nuclear ribonucleoprotein SmD1 interplays with splicing, RNA quality control, and posttranscriptional gene silencing in Arabidopsis. Plant Cell 28(2):426–438
Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, Ellacott GA, Watson JM, Wang MB, Brosnan C, Carroll BJ, Waterhouse PM (2006) RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep 7(11):1168–1175
Gazzani S, Lawrenson T, Woodward C, Headon D, Sablowski R (2004) A link between mRNA turnover and RNA interference in Arabidopsis. Science (New York, NY) 306(5698):1046–1048
Goodall GJ, Filipowicz W (1991) Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot and dicot plants. EMBO J 10(9):2635–2644
Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science (New York, NY) 286(5441):950–952
Hoffer P, Ivashuta S, Pontes O, Vitins A, Pikaard C, Mroczka A, Wagner N, Voelker T (2011) Posttranscriptional gene silencing in nuclei. Proc Natl Acad Sci USA 108(1):409–414
Huang Y, Carmichael GG (1996) Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol 16(4):1534–1542
Kanno T, Lin WD, Fu JL, Chang CL, Matzke AJM, Matzke M (2017) A genetic screen for pre-mRNA splicing mutants of Arabidopsis thaliana identifies putative U1 snRNP components RBM25 and PRP39a. Genetics 207(4):1347–1359
Katsarou K, Mavrothalassiti E, Dermauw W, Van Leeuwen T, Kalantidis K (2016) Combined activity of DCL2 and DCL3 is crucial in the defense against potato spindle tuber viroid. PLoS Pathog 12(10):e1005936
Kim YK, Kim VN (2007) Processing of intronic microRNAs. EMBO J 26(3):775–783
Koscianska E, Kalantidis K, Wypijewski K, Sadowski J, Tabler M (2005) Analysis of RNA silencing in agroinfiltrated leaves of Nicotiana benthamiana and Nicotiana tabacum. Plant Mol Biol 59(4):647–661
Kumakura N, Takeda A, Fujioka Y, Motose H, Takano R, Watanabe Y (2009) SGS3 and RDR6 interact and colocalize in cytoplasmic SGS3/RDR6-bodies. FEBS Lett 583(8):1261–1266. https://doi.org/10.1016/j.febslet.2009.03.055
Ling Y, Alshareef S, Butt H, Lozano-Juste J, Li L, Galal AA, Moustafa A, Momin AA, Tashkandi M, Richardson DN, Fujii H, Arold S, Rodriguez PL, Duque P, Mahfouz MM (2017) Pre-mRNA splicing repression triggers abiotic stress signaling in plants. Plant J 89(2):291–309
Liu HX, Goodall GJ, Kole R, Filipowicz W (1995) Effects of secondary structure on pre-mRNA splicing: hairpins sequestering the 5′ but not the 3′ splice site inhibit intron processing in Nicotiana plumbaginifolia. EMBO J 14(2):377–388
Luo Z, Chen Z (2007) Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 19(3):943–958
Manavella PA, Koenig D, Weigel D (2012) Plant secondary siRNA production determined by microRNA-duplex structure. Proc Natl Acad Sci USA 109(7):2461–2466
Martinez de Alba AE, Moreno AB, Gabriel M, Mallory AC, Christ A, Bounon R, Balzergue S, Aubourg S, Gautheret D, Crespi MD, Vaucheret H, Maizel A (2015) In plants, decapping prevents RDR6-dependent production of small interfering RNAs from endogenous mRNAs. Nucleic Acids Res 43(5):2902–2913
McHale M, Eamens AL, Finnegan EJ, Waterhouse PM (2013) A 22-nt artificial microRNA mediates widespread RNA silencing in Arabidopsis. Plant J 76(3):519–529
Mette MF, van der Winden J, Matzke MA, Matzke AJ (1999) Production of aberrant promoter transcripts contributes to methylation and silencing of unlinked homologous promoters in trans. EMBO J 18(1):241–248
Milne I, Stephen G, Bayer M, Cock PJ, Pritchard L, Cardle L, Shaw P, Marshall D (2013) Using Tablet for visual exploration of second-generation sequencing data. Brief Bioinform 14:193–202. https://doi.org/10.1093/bib/bbs012
Mlotshwa S, Pruss GJ, Gao Z, Mgutshini NL, Li J, Chen X, Bowman LH, Vance V (2010) Transcriptional silencing induced by Arabidopsis T-DNA mutants is associated with 35S promoter siRNAs and requires genes involved in siRNA-mediated chromatin silencing. Plant J 64(4):699–704
Nakasugi K, Crowhurst RN, Bally J, Wood CC, Hellens RP, Waterhouse PM (2013) De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS One 8(3):e59534
Narasimhulu SB, Deng XB, Sarria R, Gelvin SB (1996) Early transcription of Agrobacterium T-DNA genes in tobacco and maize. Plant Cell 8(5):873–886
Navarro B, Pantaleo V, Gisel A, Moxon S, Dalmay T, Bisztray G, Di Serio F, Burgyan J (2009) Deep sequencing of viroid-derived small RNAs from grapevine provides new insights on the role of RNA silencing in plant-viroid interaction. PLoS One 4(11):e7686
Parent JS, Bouteiller N, Elmayan T, Vaucheret H (2015a) Respective contributions of Arabidopsis DCL2 and DCL4 to RNA silencing. Plant J 81(2):223–232. https://doi.org/10.1111/tpj.12720
Parent JS, Jauvion V, Bouche N, Beclin C, Hachet M, Zytnicki M, Vaucheret H (2015b) Post-transcriptional gene silencing triggered by sense transgenes involves uncapped antisense RNA and differs from silencing intentionally triggered by antisense transgenes. Nucleic Acids Res 43(17):8464–8475. https://doi.org/10.1093/nar/gkv753
Polydore S, Axtell MJ (2018) Analysis of RDR1/RDR2/RDR6-independent small RNAs in Arabidopsis thaliana improves MIRNA annotations and reveals unexplained types of short interfering RNA loci. Plant J 94(6):1051–1063
Salomon S, Puchta H (1998) Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J 17(20):6086–6095
Schwach F, Moxon S, Moulton V, Dalmay T (2009) Deciphering the diversity of small RNAs in plants: the long and short of it. Brief Funct Genom Proteom 8(6):472–481
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22(12):1567–1572
Taochy C, Gursanscky NR, Cao J, Fletcher SJ, Dressel U, Mitter N, Tucker MR, Koltunow AMG, Bowman JL, Vaucheret H, Carroll BJ (2017) A genetic screen for impaired systemic RNAi highlights the crucial role of DICER-LIKE 2. Plant Physiol 175(3):1424–1437
Tinland B, Hohn B, Puchta H (1994) Agrobacterium tumefaciens transfers single-stranded transferred DNA (T-DNA) into the plant cell nucleus. Proc Natl Acad Sci USA 91(17):8000–8004
Vaistij FE, Jones L, Baulcombe DC (2002) Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell 14(4):857–867
van Kregten M, de Pater S, Romeijn R, van Schendel R, Hooykaas PJ, Tijsterman M (2016) T-DNA integration in plants results from polymerase-theta-mediated DNA repair. Nat Plants 2(11):16164
Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20(7):759–771. https://doi.org/10.1101/gad.1410506
Vermeersch L, De Winne N, Depicker A (2010) Introns reduce transitivity proportionally to their length, suggesting that silencing spreads along the pre-mRNA. Plant J 64(3):392–401
Vogt U, Pelissier T, Putz A, Razvi F, Fischer R, Wassenegger M (2004) Viroid-induced RNA silencing of GFP-viroid fusion transgenes does not induce extensive spreading of methylation or transitive silencing. Plant J 38(1):107–118
Voinnet O (2008) Post-transcriptional RNA silencing in plant-microbe interactions: a touch of robustness and versatility. Curr Opin Plant Biol 11(4):464–470
Voinnet O, Baulcombe DC (1997) Systemic signalling in gene silencing. Nature 389(6651):553
Wassenegger M, Krczal G (2006) Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci 11(3):142–151. https://doi.org/10.1016/j.tplants.2006.01.003
Wassenegger M, Heimes S, Riedel L, Sanger HL (1994) RNA-directed de novo methylation of genomic sequences in plants. Cell 76(3):567–576
Wu YY, Hou BH, Lee WC, Lu SH, Yang CJ, Vaucheret H, Chen HM (2017) DCL2- and RDR6-dependent transitive silencing of SMXL4 and SMXL5 in Arabidopsis dcl4 mutants causes defective phloem transport and carbohydrate over-accumulation. Plant J 90(6):1064–1078
Yang SJ, Carter SA, Cole AB, Cheng NH, Nelson RS (2004) A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc Natl Acad Sci USA 101(16):6297–6302
Acknowledgements
We are thankful to Dr. Guenther Buchholz for providing the plasmid pBAM-dTOM and Mario Braun for providing the plant codon-optimized dTOMATO sequence. This project was supported by the German Research Foundation (DFG; Grant WA 1019/14-1).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dalakouras, A., Lauter, A., Bassler, A. et al. Transient expression of intron-containing transgenes generates non-spliced aberrant pre-mRNAs that are processed into siRNAs. Planta 249, 457–468 (2019). https://doi.org/10.1007/s00425-018-3015-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-3015-6