Abstract
Main conclusion
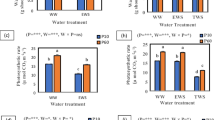
Under combined drought and mechanical stresses, mechanical stress primarily controlled physiological responses of maize. Piriformospora indica mitigated the adverse effects of stresses, and inoculated maize experienced less oxidative damage and had better adaptation to stressful conditions.
The objective of this study was to investigate the effect of maize root colonization by an endophytic fungus P. indica on plant water status, physiological traits and root morphology under combined drought and mechanical stresses. Seedlings of inoculated and non-inoculated maize (Zea mays L., cv. single cross 704) were cultivated in growth chambers filled with moistened siliceous sand at a matric suction of 20 hPa. Drought stress was induced using PEG 6000 solution with osmotic potentials of 0, − 0.3 and − 0.5 MPa. Mechanical stress (i.e., penetration resistances of 1.05, 4.23 and 6.34 MPa) was exerted by placing weights on the surface of the sand medium. After 30 days, leaf water potential (LWP) and relative water content (RWC), root and shoot fresh weights, root volume (RV) and diameter (RD), leaf proline content, leaf area (LA) and catalase (CAT) and ascorbate peroxidase (APX) activities were measured. The results show that exposure to individual drought and mechanical stresses led to higher RD and proline content and lower plant biomass, RV and LA. Moreover, increasing drought and mechanical stress severity increased APX activity by about 1.9- and 3.1-fold compared with the control. When plants were exposed to combined stresses, mechanical stress played the dominant role in controlling plant responses. P. indica-inoculated plants are better adapted to individual and combined stresses. The inoculated plants had greater RV, LA, RWC, LWP and proline content under stressful conditions. In comparison with non-inoculated plants, inoculated plants showed lower CAT and APX activities which means that they experienced less oxidative stress induced by stressful conditions.





Similar content being viewed by others
Abbreviations
- PTFE:
-
Polytetrafluoroethylene
- CM:
-
Complex medium
- DS:
-
Drought stress
- MS:
-
Mechanical stress
- Q p :
-
Penetration resistance
- RFW:
-
Root fresh weight
- SFW:
-
Shoot fresh weight
- RD:
-
Root diameter
- RV:
-
Root volume
- LA:
-
Leaf area
- RCP:
-
Root colonization percent
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- RWC:
-
Relative water content
- LWP:
-
Leaf water potential
- ANOVA:
-
Analysis of variance
References
Abadi VAJM, Sepehri M (2016) Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis 69:9–19. https://doi.org/10.1007/s13199-015-0361-z
Alameda D, Villar R (2012) Linking root traits to plant physiology and growth in Fraxinus angustifolia Vahl. seedlings under soil compaction conditions. Environ Exp Bot 79:49–57. https://doi.org/10.1016/j.envexpbot.2012.01.004
Ansari MW, Trivedi DK, Sahoo RK, Gill SS, Tuteja N (2013) A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol Biochem 70:403–410. https://doi.org/10.1016/j.plaphy.2013.06.005
Armengaud P, Thiery L, Buhot N, Grenier-de March G, Savouré A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450. https://doi.org/10.1111/j.0031-9317.2004.00251.x
Asgarzadeh H, Mosaddeghi MR, Dexter AR, Mahboubi AA, Neyshabouri MR (2014) Determination of soil available water for plants: consistency between laboratory and field measurements. Geoderma 226:8–20. https://doi.org/10.1016/j.geoderma.2014.02.020
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schäfer P, Schwarczinger I, Zuccaro A, Skoczowski A (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180:501–510. https://doi.org/10.1111/j.1469-8137.2008.02583.x
Banhara A, Ding Y, Kühner R, Zuccaro A, Parniske M (2015) Colonization of root cells and plant growth promotion by Piriformospora indica occurs independently of plant common symbiosis genes. Front Plant Sci 6:667. https://doi.org/10.3389/fpls.2015.00667
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bayat F, Mirlohi AF, Khodambashi M (2009) Effects of endophytic fungi on some drought tolerance mechanisms of tall fescue in a hydroponics culture. Russ J Plant Physiol 56:510–516. https://doi.org/10.1134/S1021443709040104
Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot 62(1):59–68. https://doi.org/10.1093/jxb/erq350
Borgo L, Marur CJ, Vieira LGE (2015) Effects of high proline accumulation on chloroplast and mitochondrial ultrastructure and on osmotic adjustment in tobacco plants. Acta Sci Agron 37:191–199. https://doi.org/10.4025/actasciagron.v37i2.19097
Caverzan A, Casassola A, Patussi Brammer SP (2016) Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In: Shanker A (ed) Abiotic and biotic stress in plants-recent advances and future perspectives. InTech, Rijeka, pp 463–480
Daryanto S, Wang L, Jacinthe PA (2016) Global synthesis of drought effects on maize and wheat production. PLoS One 11:e0156362. https://doi.org/10.1371/journal.pone.0156362
Dexter AR (1987) Mechanics of root growth. Plant Soil 98:303–312. https://doi.org/10.1007/BF02378351
Dexter AR (1990) Changes in the matric potential of soil water with time after disturbance by moulding. Soil Tillage Res 16:35–50. https://doi.org/10.1016/0167-1987(90)90020-E
Dhindsa RS, Dhindsa P, Thorpe T (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Eshel A (2013) Plant roots: the hidden half, 4th edn. CRC Press, Boca Raton
Eslami A, Fellenius BH (1997) Pile capacity by direct CPT and CPTu methods applied to 102 case histories. Can Geotech J 34:886–904. https://doi.org/10.1139/t97-056
Fakhro A, Andrade-Linares DR, von Bargen S, Bandte M, Büttner C, Grosch R, Schwarz D, Franken P (2010) Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 20:191–200. https://doi.org/10.1007/s00572-009-0279-5
FAO (2016) Food outlook: biannual report on global food markets. http://www.fao.org/3/a-i5703e.pdf
Farrar K, Bryant D, Cope-Selby N (2014) Understanding and engineering beneficial plant–microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12:1193–1206. https://doi.org/10.1111/pbi.12279
Friesen ML, Porter SS, Stark SC, von Wettberg EJ, Sachs JL, Martinez-Romero E (2011) Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst 42:23–46. https://doi.org/10.1146/annurev-ecolsys-102710-145039
Ghaffari MR, Ghabooli M, Khatabi B, Hajirezaei MR, Schweizer P, Salekdeh GH (2016) Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol Biol 90:699–717. https://doi.org/10.1007/s11103-016-0461-z
Ghannoum O (2009) C4 photosynthesis and water stress. Ann Bot 103:635–644. https://doi.org/10.1093/aob/mcn093
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, Ansari AA, Johri AK, Prasad R, Pereira E, Varma A, Tuteja N (2016) Piriformospora indica: potential and significance in plant stress tolerance. Front Microbiol 7:332. https://doi.org/10.3389/fmicb.2016.00332
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15. https://doi.org/10.6064/2012/963401
Goh CH, Vallejos DFV, Nicotra AB, Mathesius U (2013) The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J Chem Ecol 39:826–839. https://doi.org/10.1007/s10886-013-0326-8
Gohari A, Eslamian S, Abedi-Koupaei J, Bavani AM, Wang D, Madani K (2013) Climate change impacts on crop production in Iran’s Zayandeh-Rud River Basin. Sci Total Environ 442:405–419. https://doi.org/10.1016/j.scitotenv.2012.10.029
Greacen EL, Oh JS (1972) Physics of root growth. Nat New Biol 235:24–25. https://doi.org/10.1038/newbio235024a0
Grzesiak MT, Janowiak F, Szczyrek P, Kaczanowska K, Ostrowska A, Rut G, Hura T, Rzepka A, Grzesiak S (2016) Impact of soil compaction stress combined with drought or waterlogging on physiological and biochemical markers in two maize hybrids. Acta Physiol Plant 38:1–15. https://doi.org/10.1007/s11738-016-2128-4
Harrington JT, Mexal JG, Fisher JT (1994) Volume displacement provides a quick and accurate way to quantify new root production. Tree Plant Notes 45:121–124
Hill TW, Käfer E (2001) Improved protocols for aspergillus medium: elements and minimum salt stock solutions. Fungal Genet News Lett 48:20–21
Hosseini F (2015) Effect of endophytic fungus-plant symbiosis on soil water availability and physical properties, and plant growth under drought and mechanical stresses. Ph.D. Dissertation, Isfahan University of Technology (in Persian with English abstract)
Hosseini F, Mosaddeghi MR, Dexter AR (2017) Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem 118:107–120. https://doi.org/10.1016/j.plaphy.2017.06.005
Iijima M, Kato J (2007) Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Prod Sci 10:451–459. https://doi.org/10.1626/pps.10.451
Jin K, Shen J, Ashton RW, White RP, Dodd IC, Phillips AL, Parry MAJ, Whalley WR (2015) The effect of impedance to root growth on plant architecture in wheat. Plant Soil 392:323–332. https://doi.org/10.1007/s11104-015-2462-0
Jogawat A, Vadassery J, Verma N, Oelmüller R (2016) PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Nat Publ Gr 6:1–15. https://doi.org/10.1038/srep36765
Khan Z, Rho H, Firrincieli A, Hung SH, Luna V, Masciarelli O, Kim S, Doty SL (2016) Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr Plant Biol 6:38–47. https://doi.org/10.1016/j.cpb.2016.08.001
Kirkham MB (2014) Principles of soil and plant water relations, 2nd edn. Elsevier Academic Press, Amsterdam
Kumar M, Yadav V, Tuteja N, Johri AK (2009) Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 155:780–790. https://doi.org/10.1099/mic.0.019869-0
Li Z, Wu N, Liu T, Chen H, Tang M (2015) Effect of arbuscular mycorrhizal inoculation on water status and photosynthesis of Populus cathayana males and females under water stress. Physiol Plant 155:192–204. https://doi.org/10.1111/ppl.12336
Li L, Li L, Wang X, Zhu P, Wu H, Qi S (2017) Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol Biochem 119:211–223. https://doi.org/10.1016/j.plaphy.2017.08.029
Lisar SY, Motafakkerazad R, Hossain MM, Rahman IMM (2012) Water stress in plants: causes, effects and responses. In: Ismail MD, Moizur R, Hiroshi H (eds) Water stress. InTech Publ, Rijeka, pp 1–15
Lu Z, Neumann PM (1998) Water-stressed maize, barley and rice seedlings show species diversity in mechanisms of leaf growth inhibition. J Exp Bot 49:1945–1952. https://doi.org/10.1093/jxb/49.329.1945
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940. https://doi.org/10.2135/cropsci2000.404923x
Materechera SA, Dexter AR, Alston AM (1991) Penetration of very strong soils by seedling roots of different plant species. Plant Soil 135:31–41. https://doi.org/10.1007/BF00014776
Mckee GW (1964) A coefficient for computing leaf area in hybrid corn. Agron J 56:240–241
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916. https://doi.org/10.1104/pp.51.5.914
Mirreh HF, Ketcheson JW (1973) Influence of soil water matric potential and resistance to penetration on corn root elongation. Can J Soil Sci 53:383–388. https://doi.org/10.4141/cjss73-055
Mwenye OJ, Van Rensburg L, Van Biljon A, Van der Merwe R (2016) The role of proline and root traits on selection for drought-stress tolerance in soybeans: a review. S Afr J Plant Soil 33:245–256. https://doi.org/10.1080/02571862.2016.1148786
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach choloroplast. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53:988–998. https://doi.org/10.1111/j.1365-313X.2007.03390.x
Orcutt DM, Nilsen ET (2000) The physiology of plants under stress: soil and biotic factors. Wiley, New York, p 680
Passioura JB (2002) Soil conditions and plant growth. Plant Cell Environ 25:311–318. https://doi.org/10.1046/j.0016-8025.2001.00802.x
Peškan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost G, Varma A, Oelmüller R (2004) Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant–microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 122:465–477. https://doi.org/10.1111/j.1399-3054.2004.00424.x
Rahimzadeh S, Pirzad A (2017) Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): a field study. Mycorrhiza 10:1–6. https://doi.org/10.1007/s00572-017-0775-y
Rai M, Acharya D, Singh A, Varma A (2001) Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza 11:123–128. https://doi.org/10.1007/s005720100115
Romanello GA, Chuchra-Zbytniuk KL, Vandermer JL, Touchette BW (2008) Morphological adjustments promote drought avoidance in the wetland plant Acorus americanus. Aquat Bot 89:390–396. https://doi.org/10.1016/j.aquabot.2008.04.007
Saia S, Amato G, Frenda AS, Giambalvo D, Ruisi P (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9:e90738. https://doi.org/10.1371/journal.pone.0090738
Sherameti I, Venus Y, Drzewiecki C, Tripathi S, Dan VM, Nitz I, Varma A, Grundler FM, Oelmüller R (2008) PYK10, a β-glucosidase located in the endoplasmatic reticulum, is crucial for the beneficial interaction between Arabidopsis thaliana and the endophytic fungus Piriformospora indica. Plant J 54:428–439. https://doi.org/10.1111/j.1365-313X.2008.03424.x
Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B (2010) Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol 167:1009–1017. https://doi.org/10.1016/j.jplph.2010.02.013
Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kannaste A, Behers L, Nevo E, Seisenbaeva G, Stenstrom E, Niinements U (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9:e96086. https://doi.org/10.1371/journal.pone.0096086
Touchette BW, Smith GA, Rhodes KL, Poole M (2009) Tolerance and avoidance: two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. J Exp Mar Biol Ecol 380:106–112. https://doi.org/10.1016/j.jembe.2009.08.015
Varma A, Verma S, Sudh A, Sahay N, Butehron B, Franken P (1999) Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 65:2741–2744
Veen BW, Boone FR (1990) The influence of mechanical resistance and soil-water on the growth of seminal roots of maize. Soil Tillage Res 16:219–226. https://doi.org/10.1016/0167-1987(90)90031-8
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759. https://doi.org/10.1007/s00726-008-0061-6
Vierheilig H, Schweiger P, Brundrett M (2005) An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol Plant 125:393–404. https://doi.org/10.1111/j.1399-3054.2005.00564.x
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102:13386–13391. https://doi.org/10.1073/pnas.0504423102
Whalley WR, Clark LJ (2011) Drought stress, effect on soil mechanical impedance and root (crop) growth. In: Glinski J, Horabik J, Lipiec J (eds) Encyclopedia of agrophysics. Springer Press, Dordrecht, pp 228–231. https://doi.org/10.1007/978-90-481-3585-1_43
Whalley WR, Watts CW, Gregory AS, Mooney SJ, Clark LJ, Whitmore AP (2008) The effect of soil strength on the yield of wheat. Plant Soil 306:237–247. https://doi.org/10.1007/s11104-008-9577-5
Xiao X, Yang F, Zhang S, Korpelainen H, Li C (2009) Physiological and proteomic responses of two contrasting Populus cathayana populations to drought stress. Physiol Plant 136:150–168. https://doi.org/10.1111/j.1399-3054.2009.01222.x
Zarea MJ, Hajinia S, Karimi N, Goltapeh EM, Rejali F, Varma A (2012) Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol Biochem 45:139–146. https://doi.org/10.1016/j.soilbio.2011.11.006
Zlatev Z, Lidon FC (2012) An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir J Food Agric 24:57–72
Acknowledgements
We would like to thank Iran National Science Foundation (INSF) and Isfahan University of Technology for the award of a Postdoctoral Research Fellowship (no. 94027490) to the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hosseini, F., Mosaddeghi, M.R., Dexter, A.R. et al. Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta 247, 1229–1245 (2018). https://doi.org/10.1007/s00425-018-2861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2861-6