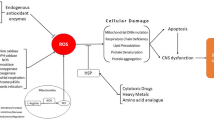
Abstract
At normal aging, the brain exhibits signs of compromised bioenergetic and increased levels of products of interaction between reactive oxygen/nitrogen species (ROS/RNS) and brain constituents. Under normal conditions, steady-state levels of ATP and ROS/RNS fluctuate in certain ranges providing basis for stable homeostasis. However, from time to time these parameters leave a “comfort zone,” and at adulthood, organisms are able to cope with these challenges efficiently, whereas at aging, efficiency of the systems maintaining homeostasis declines. That is very true for the brain due to high ATP demands which are mainly covered by mitochondrial oxidative phosphorylation. Such active oxidative metabolism gives rise to intensive ROS generation as side products. The situation is worsened by high brain level of polyunsaturated fatty acids which are substrates for ROS/RNS attack and production of lipid peroxides. In this review, organization of energetic metabolism in the brain with a focus on its interplay with ROS at aging is discussed. The working hypothesis on aging as a disbalance between oxidative stress and energy provision as a reason for brain aging is proposed. From this point of view, normal age-related physiological decline in the brain functions results from increased disbalance between decrease in capability of the brain to control constantly increased incapability to maintain ROS levels and produce ATP due to amplification of vicious cycles intensification of oxidative stress <----> impairment of energy provision.


Similar content being viewed by others
Data Availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- AcCoA:
-
Acetyl coenzyme A
- ETC:
-
Electron transport chain
- KB:
-
Ketone bodies
- LPO:
-
Lipid peroxidation
- NMR:
-
Nuclear magnetic resonance
- OxPhos:
-
Oxidative phosphorylation
- PPP:
-
Pentose phosphate pathway
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- TCA cycle:
-
Tricarboxylic acid cycle
References
Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS (2002) Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab 22(3):271–279. doi:https://doi.org/10.1097/00004647-200203000-00004
Auestad N, Korsak RA, Morrow JW, Edmond J (1991) Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 56(4):1376–1386. https://doi.org/10.1111/j.1471-4159.1991.tb11435.x
Berndt N, Bulik S, Holzhütter H-G (2012) Kinetic modeling of the mitochondrial energy metabolism of neuronal cells: the impact of reduced α-ketoglutarate dehydrogenase activities on ATP production and generation of reactive oxygen species. Int J Cell Biol 2012:757594–757511. https://doi.org/10.1155/2012/757594
Brandes MS, Gray NE (2020) NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro 12:1–23. https://doi.org/10.1177/1759091419899782
Butterfield DA (2020) Brain lipid peroxidation and alzheimer disease: synergy between the Butterfield and Mattson laboratories. Ageing Res Rev In press 101049:101049. https://doi.org/10.1016/j.arr.2020.101049
Butterfield DA, Hardas SS, Lange MLB (2010) Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer disease: many pathways to neurodegeneration. J Alzheimers Dis 20(2):369–393. https://doi.org/10.3233/JAD-2010-1375
Cai Z, Yan L, Li K et al (2012) Roles of AMP-activated protein kinase in Alzheimer's disease. Neuromol Med 14:1–14. https://doi.org/10.1007/s12017-012-8173-2
Cantu D, Schaack J, Patel M (2009) Oxidative inactivation of mitochondrial aconitase results in iron and H2O2-mediated neurotoxicity in rat primary mesencephalic cultures. PLoS One 4(9):e7095. https://doi.org/10.1371/journal.pone.0007095
Chen R, Lai UH, Zhu L, Singh A, Ahmed M, Forsyth NR (2018) Reactive oxygen species formation in the brain at different oxygen levels: the role of hypoxia inducible factors. Front Cell Dev Biol 6: 132. https://doi.org/10.3389/fcell.2018.00132
Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di PM, Villani G (2005) Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med 38:796–805. https://doi.org/10.1016/j.freeradbiomed.2004.11.034
Cunnane S, Nugent S, Roy M (2011) Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 27(1):3–20. https://doi.org/10.1016/j.nut.2010.07.021
Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano C-A (2016) Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci 1367(1):12–20. https://doi.org/10.1111/nyas.12999
De Backer I, Hussain SS, Bloom SR, Gardiner JV (2016) Insights into the role of neuronal glucokinase. Am J Physiol Endocrinol Metab 311(1):E42–E55. https://doi.org/10.1152/ajpendo.00034.2016
Deus CM, Yambire KF, Oliveira PJ, Raimundo N (2020) Mitochondria-lysosome crosstalk: from physiology to neurodegeneration. Trends Mol Med. 26(1):71–88. https://doi.org/10.1016/j.molmed.2019.10.009
De Vries MG, Arseneau LM, Lawson ME, Beverly JL (2003) Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52(11):2767–2773. https://doi.org/10.2337/diabetes.52.11.2767
Domise M, Didier S, Marinangeli C, Zhao H, Chandakkar P, Buée L, Viollet B, Davies P, Marambaud P, Vingtdeux V (2016) AMP-activated protein kinase modulates tau phosphorylation and tau pathology in vivo. Sci Rep 6:26758. https://doi.org/10.1038/srep26758
Dong Y, Brewer GJ (2019) Global metabolic shifts in age and Alzheimer's disease mouse brains pivot at NAD+/NADH redox sites. J Alzheimers Dis 71(1):119–140. https://doi.org/10.3233/JAD-190408
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623(2):208–214. https://doi.org/10.1016/0006-8993(93)91429-V
Duarte JM, Do KQ, Gruetter R (2014) Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging 35(7):1660–1668. https://doi.org/10.1016/j.neurobiolaging.2014.01.135
El-Sawalhi MM, Darwish HA, Mausouf MN, Shaheen AA (2013) Modulation of age-related changes in oxidative stress markers and energy status in the rat heart and hippocampus: a significant role for ozone therapy. Cell Biochem Funct 31(6):518–525. https://doi.org/10.1002/cbf.2930
Erecinska M, Silver IA (1989) ATP and brain function. J Cereb Blood Flow Metab 9(1):2–19. https://doi.org/10.1038/jcbfm.1989.2
Forester BP, Berlow YA, Harper DG, Jensen JE, Lange N, Froimowitz MP, Ravichandran C, Iosifescu DV, Lukas SE, Renshaw PF, Cohen BM (2010) Age-related changes in brain energetic and phospholipid metabolism. NMR Biomed 23(3):242–250. https://doi.org/10.1002/nbm.1444
Garaschuk O, Semchyshyn HM, Lushchak VI (2018) Healthy brain aging: interplay between reactive species, inflammation and energy supply. Ageing Res Rev 43:26–45. https://doi.org/10.1016/j.arr.2018.02.003
Gaspar JM, Velloso LA (2018) Hypoxia inducible factor as a central regulator of metabolism – implications for the development of obesity. Front Neurosci 12:813. https://doi.org/10.3389/fnins.2018.00813
Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem 143(4):418–431. https://doi.org/10.1111/jnc.14037
Guzmán M, Blázquez C (2004) Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids 70(3):287–292. https://doi.org/10.1016/j.plefa.2003.05.001
Haripriya D, Devi MA, Kokilavani V, Sangeetha P, Panneerselvam C (2004) Age dependent alterations in mitochondrial enzymes in cortex, striatum and hippocampus of rat brain – potential role of L-carnitine. Biogerontology 5(5):355–364. https://doi.org/10.1007/s10522-004-2575-y
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Harris RA, Tindale L, Lone A, Singh O, Macauley SL, Stanley M, Holtzman DM, Bartha R, Cumming RC (2016) Aerobic glycolysis in the frontal cortex correlates with memory performance in wild-type mice but not the APP/PS1 mouse model of cerebral amyloidosis. J Neurosci 36(6):1871–1878. https://doi.org/10.1523/JNEUROSCI.3131-15.2016
Hunsberger HC, Greenwood BP, Tolstikov V, Narain NR, Kiebish MA, Denny CA (2020) Divergence in the metabolome between natural aging and Alzheimer's disease. Sci Rep 10:12171. https://doi.org/10.1038/s41598-020-68739-z
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8:9–10. https://doi.org/10.1089/ars.2006.8.1583
Ivanisevic J, Stauch KL, Petrascheck M, Benton HP, Epstein AA, Fang M, Gorantla S, Tran M, Hoang L, Kurczy ME, Boska MD, Gendelman HE, Fox HS, Siuzdak G (2016) Metabolic drift in the aging brain. Aging (Albany, NY) 8(5):1000–1020. https://doi.org/10.18632/aging.100961
Kaiser LG, Hirokazu K, Fukunaga M, Matson GB (2016) Detection of glucose in the human brain with 1H MRS at 7 Tesla. Magn Reson Med 76:1653–1660. https://doi.org/10.1002/mrm.26456
Karaca M, Frigerio F, Migrenne S, Martin-Levilain J, Skytt DM, Pajecka K, Martin-del-Rio R, Gruetter R, Tamarit-Rodriguez J, Waagepetersen HS, Magnan C, Maechler P (2015) GDH-dependent glutamate oxidation in the brain dictates peripheral energy substrate. Cell Rep 13(2):365–375. https://doi.org/10.1016/j.celrep.2015.09.003
Koepsell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch - Eur J Physiol. 472:1299–1343. https://doi.org/10.1007/s00424-020-02441-x
Le Foll C, Levin BE (2016) Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol 310(11):R1186-R1192. https://doi.org/10.1152/ajpregu.00113.2016, 310
Lesnefsky EJ, Hoppel CL (2006) Oxidative phosphorylation and aging. Ageing Res Rev 5(4):402–433. https://doi.org/10.1016/j.arr.2006.04.001
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36(9:1495–1502. https://doi.org/10.1016/S0531-5565(01)00135-8
Liemburg-Apers DC, Willems PHGM, Koopman WJH, Grefte S (2015) Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol 89:1209–1226. https://doi.org/10.1007/s00204-015-1520-y
Lushchak OV, Piroddi M, Galli F, Lushchak VI (2014) Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep 19(1):8–15. https://doi.org/10.1179/1351000213Y.0000000073
Lushchak VI (2007) Free radical oxidation of proteins and its relationship with functional state of organisms. Biochemistry (Mosc) 72(8):809–827. https://doi.org/10.1134/S0006297907080020
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron. 86(4):883–901. https://doi.org/10.1016/j.neuron.2015.03.035
Martínez de Morentin PB, Urisarri A, Couce ML, López M (2016) Molecular mechanisms of appetite and obesity: a role for brain AMPK. Clin Sci (Lond) 130(19):1697–1709. https://doi.org/10.1042/CS20160048
Mason S (2017) Lactate shuttles in neuroenergetics—homeostasis, allostasis and beyond. Front Neurosci 11:43. https://doi.org/10.3389/fnins.2017.00043
Mattson MP (2012) Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 16(6):706–722. https://doi.org/10.1016/j.cmet.2012.08.012
Mattson MP, Arumugam TV (2018) Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab 27(6):1176–1199. https://doi.org/10.1016/j.cmet.2018.05.011
McKenna MC, Scafidi S, Robertson CL (2015) Metabolic alterations in developing brain after injury: knowns and unknowns. Neurochem Res 40:2527–2543. https://doi.org/10.1007/s11064-015-1600-7
Melo HM, Santos LE, Ferreira ST (2019) Diet-derived fatty acids, brain inflammation, and mental health. Front Neurosci 13:265. https://doi.org/10.3389/fnins.2019.00265
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34(2-3):121–138. https://doi.org/10.1016/j.mam.2012.07.001
Nakajima S, Kunugi H (2020) Lauric acid promotes neuronal maturation mediated by astrocytes in primary cortical cultures. Heliyon 6(5):e03892. https://doi.org/10.1016/j.heliyon.2020.e03892
Naudí A, Caro P, Jové M, Gómez J, Boada J, Ayala V, Portero-Otín M, Barja G, Pamplona R (2007) Methionine restriction decreases endogenous oxidative molecular damage and increases mitochondrial biogenesis and uncoupling protein in rat brain. Rejuvenation Res 10(4):473–484. https://doi.org/10.1089/rej.2007.0538
Nissanka N, Moraes CT (2018) Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett 592:728–742. https://doi.org/10.1002/1873-3468.12956
Ogunnowo-Bada EO, Heeley N, Brochard L, Evans ML (2014) Brain glucose sensing, glucokinase and neural control of metabolism and islet function. Diabetes Obes Metab 16(1):26–32. https://doi.org/10.1111/dom.12334
Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrnea E (1999) Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 111(1):39–47. https://doi.org/10.1016/S0047-6374(99)00071-8
Paolino N, Massimiliano D, Samuela C, Enrica B (2001) Rabbit brain glucose-6-phosphate dehydrogenase: biochemical properties and inactivation by free radicals and 4-hydroxy-2-nonenal. Neuroreport 12(18):4149–4153. https://doi.org/10.1097/00001756-200112210-00057
Phyu S, Tseng C, Fleming I et al (2016) Probing the PI3K/Akt/mTor pathway using 31P-NMR spectroscopy: routes to glycogen synthase kinase 3. Sci Rep 6:36544. https://doi.org/10.1038/srep36544
Quijano C, Trujillo M, Castro L, Trostchansky A (2016) Interplay between oxidant species and energy metabolism. Redox Biol 8:28–42. https://doi.org/10.1016/j.redox.2015.11.010
Raz N, Daugherty AM (2018) Pathways to brain aging and their modifiers: free-radical-induced energetic and neural decline in senescence (FRIENDS) model. Gerontology 64:49–57. https://doi.org/10.1159/000479508
Rich L, Brown AM (2016) Glycogen: multiple roles in the CNS. Neuroscientist 23(4):356–363. https://doi.org/10.1177/1073858416672622
Riske L, Thomas RK, Baker GB, Dursun SM (2017) Lactate in the brain: an update on its relevance to brain energy, neurons, glia and panic disorder. Ther Adv Psychopharmacol 7(2):85–89. doi:https://doi.org/10.1177/2045125316675579
Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell. 146(5):682–695. https://doi.org/10.1016/j.cell.2011.07.030
Salman M, Tabassum H, Parvez S (2020) Nrf2/HO-1 mediates the neuroprotective effects of pramipexole by attenuating oxidative damage and mitochondrial perturbation after traumatic brain injury in rats. Dis Model Mech 13(8):dmm045021. https://doi.org/10.1242/dmm.045021
Semchyshyn HM (2014) Reactive carbonyl species in vivo: generation and dual biological effects. Sci World J 2014:417842–417810. https://doi.org/10.1155/2014/417842
Sharman EH, Bondy SC (2001) Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging 22(4):629–634. https://doi.org/10.1016/S0197-4580(01)00226-3
Sies H, Jones DP (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21:363–383. https://doi.org/10.1038/s41580-020-0230-3
Silver IA, Erecinska M (1994) Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14(8):5068–5076. https://doi.org/10.1523/JNEUROSCI.14-08-05068.1994
Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF (2004) Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. Neurosci. 24(36):7779–7788. https://doi.org/10.1523/JNEUROSCI.1899-04.2004
Stefanatos R, Sanz A (2018) The role of mitochondrial ROS in the aging brain. FEBS Lett 592:743–758. https://doi.org/10.1002/1873-3468.12902
Tian L, Cai Q, Wei H (1998) Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med 24(9):1477–1484. https://doi.org/10.1016/S0891-5849(98)00025-2
Tretter L, Adam-Vizi V (2005) Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Phil Trans R Soc B 360:2335–2345. https://doi.org/10.1098/rstb.2005.1764
Walsh ME, Shi Y, Van Remmen H (2014) The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med 66:88–99. https://doi.org/10.1016/j.freeradbiomed.2013.05.037
Yan L-J, Levine RL, Sohal RS (1997) Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A 94(21):11168–11172. https://doi.org/10.1073/pnas.94.21.11168
Yin F, Sancheti H, Patil I, Cadenas E (2016) Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free Radic Biol Med 100:108–122. https://doi.org/10.1016/j.freeradbiomed.2016.04.200
Zhao Z, Yu Z, Hou Y, Zhang L, Fu A (2020) Improvement of cognitive and motor performance with mitotherapy in aged mice. Int J Biol Sci 16(5):849–858. https://doi.org/10.7150/ijbs.40886
Acknowledgments
The author would like to thank Drs. H. Semchyshyn and M. Bayliak for critical reading of the manuscript and two anonymous reviewers for their careful reading of the manuscript and their many constructive comments with suggestions that resulted in better presentation of the material.
Funding
This work was partially supported by a grant #90233 from the Volkswagen Foundation (VolkswagenStiftung, Germany) and a grant #0118U003477 from the Ministry of Education and Science of Ukraine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The author declares no conflict of interest.
Consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Aging Brain in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Lushchak, V.I. Interplay between bioenergetics and oxidative stress at normal brain aging. Aging as a result of increasing disbalance in the system oxidative stress–energy provision. Pflugers Arch - Eur J Physiol 473, 713–722 (2021). https://doi.org/10.1007/s00424-021-02531-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02531-4