Abstract
The identification of rare disease-causing variants in humans by large-scale next-generation sequencing (NGS) studies has also provided us with new insights into the pathophysiological role of de novo missense variants in the CACNA1D gene that encodes the pore-forming α1-subunit of voltage-gated Cav1.3 L-type Ca2+ channels. These CACNA1D variants have been identified somatically in aldosterone-producing adenomas as well as germline in patients with neurodevelopmental and in some cases endocrine symptoms. In vitro studies in heterologous expression systems have revealed typical gating changes that indicate enhanced Ca2+ influx through Cav1.3 channels as the underlying disease-causing mechanism. Here we summarize the clinical findings of 12 well-characterized individuals with a total of 9 high-risk pathogenic CACNA1D variants. Moreover, we propose how information from somatic mutations in aldosterone-producing adenomas could be used to predict the potential pathogenicity of novel germline variants. Since these pathogenic de novo variants can cause a channel-gain-of function, we also discuss the use of L-type Ca2+ channel blockers as a potential therapeutic option.
Similar content being viewed by others
In the past few years the identification of rare disease-causing variants in humans by large-scale next-generation sequencing (NGS) studies has provided us with unprecedented new insights into the physiological and pathophysiological role of ion channels, including voltage-gated Ca2+ channels (Cavs, Table 1). The first disease-causing genetic Cav variants were inherited conditions in CACNA1A causing familial hemiplegic migraine type 1 and episodic ataxia type 2 [61, 63] or CACNA1F causing eye disorders such as congenital stationary night blindness type 2 [84, 86, 96]. The first de novo variants in L-type Cavs were found in CACNA1C leading to Timothy syndrome, a multisystem disorder [39, 80, 81]. More recently, high-throughput sequencing of family trios and quads in well-defined disease cohorts in combination with advanced bioinformatic pipelines and the availability of large genetic databases led to the discovery of disease-causing de novo missense variants in the pore-forming α1-subunits of several Cav subtypes (Table 1). This includes variants in CACNA1E (Cav2.3 α1) causing developmental epileptic encephalopathies with contractures, macrocephaly, and dyskinesia [22], in CACNA1G (Cav3.1 α1) causing childhood-onset cerebellar atrophy [13] and in CACNA1H (Cav3.2 α1) causing early-onset hypertension with primary aldosteronism [74]. In addition, there is accumulating evidence that CACNA1D (Cav1.3 α1) variants cause an often severe neurodevelopmental syndrome, which is reviewed in this article.
Interestingly, de novo pathogenic CACNA1D variants and similarly those in CACNA1C, CACNA1E, CACNA1G, and CACNA1H are not gene-disrupting resulting in a loss of channel function. Instead, they cause typical changes of channel gating, which can enhance channel activity (gain of channel function) during electrical activity patterns in neurons, endocrine, and other electrically excitable cells. These very characteristic gating changes allow the classification of these variants as pathogenic variants in electrophysiological recordings after heterologous expression in mammalian cells (Fig. 1, Table 2). Most variants are located within regions of the pore-forming α1-subunits, which are critical for the function of the activation gate and its control by the channels’ voltage-sensors (see chapter below). Recently published cryo-electron microscopy and crystal structures of two Cav α1-subunits (Cav1.1, Cav3.1; [93, 99]) and bacterial sodium channels (BacNavs) in different states [37, 92] now enable the construction of homology models, which help us to predict how these variants interfere with basic channel functions on the molecular level. Therefore, these variants provide important insight not only into disease but also into the structure-function relationship of Cav α1-subunits.
Characteristic gating changes induced by high-risk pathogenic CACNA1D variants permitting enhanced channel function of Cav1.3 L-type Ca2+ channels (taken and modified from [66, 26]). Typical changes in the voltage dependence of activation (A+C) and inactivation (C) as well as inactivation kinetics during prolonged depolarizations (B+D) are shown for C-terminally short wild-type (black), G407R (red; type 1) and S652L Cav1.3 channels (turquoise; type 2). Note that for the almost non-inactivating G407R variant no steady-state inactivation curve was determined (A). The short Cav1.3 splice variant lacks an intramolecular interaction between the distal and proximal C-terminus which enhances its voltage-sensitivity and inactivation kinetics compared with full-length Cav1.3 channels [8, 77]; however, similar gating changes were also elicited in the long Cav1.3 variant [26, 64]
Gene disrupting de novo variants (frameshift, premature stop codon, splice-donor defect) causing loss of channel function can be reliably predicted using bioinformatic pipelines in most cases. In contrast, it is much more difficult to distinguish high-risk, disease-causing de novo missense variants, for which the functional consequences are difficult to predict in silico, from rare missense variants, which contribute only weakly to disease risk or are even benign. This has important clinical implications, because the symptomatic spectrum of a Ca2+ channelopathy should primarily be inferred from rare variants proven to confer high risk in functional studies.
In this review we summarize the spectrum of symptoms associated with a syndrome caused by de novo CACNA1D missense variants leading to aberrant gating properties of Cav1.3 Ca2+ channels, which support enhanced channel activity especially in cells firing from negative membrane potentials. We update the clinical phenotype of all well-documented pathogenic CACNA1D variants affecting a total of 12 individuals. This should help to guide clinical diagnosis and help to outline a rational strategy for potential personalized therapy with Ca2+ channel blockers.
Physiological role of Cav1.3 L-type Ca2+ channels
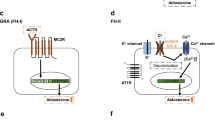
The fact that aberrant gating of Cav1.3 Ca2+ channels can cause neurodevelopmental and endocrine symptoms can be explained by the multiple functions of these channels in the mammalian organism. Of the L-type family (Cav1, Table 1), the Cav1.2 and Cav1.3 isoforms show a wide and often overlapping tissue distribution and can be found in most electrically excitable cells (for review see [96]). Cav1.3 channels, although classified as high-voltage activated (HVA, Table 1), can operate at much more negative membrane potentials compared with other HVA Cavs [40, 42], which enables them to support special functions within the auditory, cardiac, endocrine, and nervous system as outlined below. Insight into the physiological roles of Cav1.3 channels came from Cav1.3-knockout mice [54, 67] and humans harboring a mutation in the CACNA1D gene resulting in non-functional Cav1.3 channels [4]. In both loss of Cav1.3 function resulted in congenital deafness, bradycardia and sinoatrial node (SAN) arrhythmia (human SAN dysfunction and deafness syndrome, SANDD, OMIM # 614896). The hearing loss can be explained by the important role of presynaptically clustered Cav1.3 channels in cochlear hair cells where they provide Ca2+ influx to trigger neurotransmitter release at synaptic ribbons [10, 67, 83]. In contrast, neuronal Cav1.3 channels are predominantly expressed postsynaptically where they shape electrical activity patterns, contribute to dendritic Ca2+ signaling, and fine-tune Ca2+-dependent gene expression (for review see [96]). In the heart Cav1.3 channels predominate in the SAN and atrio-ventricular node where Cav1.3 Ca2+ influx at negative potentials drives the diastolic depolarization required for normal cardiac pacemaking [45, 47, 98], which explains the observed cardiac phenotype in Cav1.3-deficient humans and mice. Cav1.3 also controls endocrine functions in the pancreas and in the adrenal gland. In mice, Cav1.3 does not contribute much to the overall Ca2+ current and insulin release from pancreatic β cells [78]. However, in one Cav1.3-knockout mouse line genetic ablation of Cav1.3 induced hypoinsulinemia and impaired glucose tolerance, associated with a deficit in postnatal β cell generation/proliferation ([54], but see [67]). In human pancreatic β cells, Cav1.3 transcripts predominate and seem to be involved in exocytosis [68]. In cultured catecholamine-releasing chromaffin cells of the murine adrenal medulla, Cav1.3 Ca2+ channels mediate ~ 25% of the total Ca2+ current, support autonomous pacemaker activity [46], and shape secretion-associated firing patterns of these neuroendocrine cells [89]. Cav1.3 is also expressed in aldosterone-secreting zona glomerulosa cells of the adrenal cortex [16, 72]. In these cells, aldosterone synthesis is driven by a periodic intracellular Ca2+ signal that mainly depends on low-voltage activated Cav3.2 T-type channels; however, Cav1.3 also contributes to this Ca2+ signal (for review see [5]). These special functions of Cav1.3 channels in pancreatic β cells and aldosterone-producing cells nicely explain that individuals harboring an activity-promoting de novo CACNA1D variant (germline or somatically in aldosterone-producing adenomas, APAs) can present with primary aldosteronism and/or hyperinsulinemic hypoglycemia (see below, Table 3).
While the patients with de novo CACNA1D missense variants that are described in this review exhibit neurodevelopmental symptoms (Table 3), no brain pathologies have been reported so far for Cav1.3-deficient humans. However, using genetic and pharmacological approaches in mouse models helped to reveal several important functions of Cav1.3 in the central nervous system. Cav1.3 channels account for ~ 10% of total L-type Ca2+ channels in the brain and are expressed within multiple regions [6, 78, 79] where they can shape neuronal excitability and induce gene transcription, important for synaptic plasticity, memory formation, and neuronal development. Cav1.3-deficient mice show subtle deficits in proper brain development, evident from a lower number of dopamine-producing neurons in the substantia nigra [62] and a decreased volume and neuron number in the auditory brain stem [24, 25] and the dentate gyrus [48]. The latter was associated with reduced hippocampal neurogenesis and impairments in hippocampus-dependent cognitive functions [48]. Cav1.3 channels are also involved in mood and emotional behaviors. Loss of Cav1.3 resulted in an antidepressant-like phenotype in mice [11], while the opposite was observed upon selective Cav1.3 activation [78]. Also, stimulation of Cav1.3 within the ventral tegmental area (VTA; dopamine midbrain system) was sufficient to elicit the depressive-like behavior together with a social deficit and enhanced cocaine-associated behaviors [49]. This was in line with previous reports linking Cav1.3 activity to the development of psychostimulant-induced sensitized behaviors [20, 33, 71]. An anxiety-like phenotype upon global Cav1.3 knockout was most likely the consequence of the knockout-induced deafness [11], whereas fore-brain specific knockdown of Cav1.3 had no effect on anxiety-related parameters in mice [36]. However, the consolidation of conditioned contextual fear memory is facilitated by Cav1.3 activity, as it was impaired in Cav1.3-deficient mice and associated with significantly reduced long-term potentiation (LTP) in the basolateral amygdala [50, 51]. There is also evidence linking the oscillatory Cav1.3 Ca2+ influx in dendrites of autonomously spiking substantia nigra neurons to their selective cell death in Parkinson’s disease ([21] but see [62]).
Given the multiple functions of Cav1.3 Ca2+ channels throughout the body, it is plausible that interfering with the way these channels conduct Ca2+ ions can result in the dysregulation of several body functions and thus to human diseases.
Characteristic gating changes of Cav1.3 channels associated with high-risk pathogenic variants
The typical gating changes repeatedly observed in well-characterized patients with a pathogenic CACNA1D de novo variant have been described in previous publications (Fig. 1; for references see Table 2). It is important to note that some of these germline variants have also been reported as somatic variants in APAs ([3, 72], Table 2), which likely evolve from aldosterone-producing cell clusters (APCCs) and lesions called APCC-to-APA translational lesions in the adrenal cortex ([2, 9] for review). Since aldosterone production is Ca2+-dependent [9], this independently supports the prediction that the observed gating changes cause enhanced Ca2+ signaling through the channel. This is also important from a diagnostic standpoint because the pathogenic potential of a newly identified germline CACNA1D variant in an affected individual is always unclear, unless in vitro functional data are available. However, if a rare germline variant has also been reported as an APA/APCC variant, for which a much larger number has been identified as compared with germline variants (Fig. 2), this strongly supports its pathogenicity. We have therefore compiled all currently published CACNA1D APA/APCCs variants and will discuss them below.
Scheme of the position of de novo germline and de novo somatic APA/APCC variants within the Cav1.3 α1-subunit. The α1-subunit consists of four homologous repeats (I–IV), each comprising six transmembrane segments (S1–S6). From each repeat, S1–S4 form the voltage sensors (S4 contains the positive gating charges) and S5–S6 with their connecting loop build the ion conducting pore. The S4–S5 loop links the voltage-sensor movements to the pore opening. Color code: germline (orange), somatic (blue)
The functional characterization of a larger set of both germline and somatic variants using whole-cell patch-clamp studies by us and others [3, 26, 41, 52, 64,65,66, 72, 87] now allows to propose at least four characteristic types of functional alterations leading to a channel gain of function. These have been published recently [66] and are depicted in (Table 2).
Type 1 are the inactivation-deficient variants, such as G403D, G403R, and G407R, in which most of the Cav1.3 current fails to inactivate (Fig. 1B). They could also be classified as “Timothy syndrome like” variants because they induce similar gating changes as the Cav1.2 α1-subunit (CACNA1C) variants G402S and G406R that affect the corresponding amino acid residue and cause Timothy syndrome, a multisystem disorder also associated with autism [7, 80, 81, 96]. An example is illustrated in Fig. 1A, B for variant G407R (introduced into the short splice-variant of Cav1.3 [66]). Type 2 variants still inactivate to variable extents (i.e., faster or slower than wild-type) but are characterized by pronounced negative shifts of the voltage-dependence of activation with or without a strong negative shift also of the inactivation voltage. An example is shown in Fig. 1C, D for the recently published S652L variant [26]. Type 3 is characterized only by slower and less complete inactivation after 3–5 s at the voltage of maximal activation (Vmax), which should favor persistent current in cells during prolonged depolarizations (Table 2). Type 4 are variants in the voltage sensor (e.g., S4 helix positive charges), which enable depolarizing ω-currents as described in several other voltage-gated Ca2+ channels [12, 29, 32, 84]. For variants without a functional phenotype in vitro, we cannot exclude pathogenicity through molecular mechanisms which escape our in vitro functional analysis (such as tissue-specific protein-protein interactions disrupted by the variant). However, their role for the disease etiology remains uncertain until further functional or clinical data provide more conclusive insight (e.g., by demonstrating their presence in a larger number of patients and/or in APAs/APCCs).
Clinical characteristics of germline CACNA1D variants
Here we summarize the clinical presentation of 12 individuals with a confirmed or predicted high-risk, pathogenic CACNA1D variant for which a well-documented clinical history is available and the “diagnostic” gating changes of Cav1.3 have either been confirmed in functional studies or are strongly supported (variant A749T) by molecular modeling (see below).
The first reported variant (A749G) has been identified in an individual with autism spectrum disorder (ASD) and intellectual impairment but without evidence for additional neurological symptoms ([58, 64]; case no. 5 in Table 3). A subsequent report [72] described pathogenic CACNA1D variants in two patients with congenital primary aldosteronism, seizures, and neurological abnormalities (PASNA, OMIM # 615474; cases no. 1, no. 3 in Table 3). However, for both patients it could not be excluded that birth complications contributed to their neurological symptoms. Despite the report of another subject (G407R; case no. 6 in Table 3) affected by ASD without intellectual impairment and neurological symptoms [28, 64], the majority of variants we currently oversee leads to a severe developmental disorder also associated with developmental delay, intellectual impairment, neurological symptoms (including seizures in some of them), and, in several cases, endocrine symptoms, evident as primary aldosteronism, congenital hyperinsulinemic hypoglycemia, or both (Table 3).
Recently, two cases affected by the de novo CACNA1D variant A749T were diagnosed (cases no. 10, no. 11 in Table 3). It is located in the same position as the A749G variant described above within the “LAIA” motif discussed in more detail below. Based on its location and the nature of the amino acid exchange, we also predict pathogenicity for this variant, although this needs confirmation in functional studies as for other germline de novo variants (Table 3). Because clinical characteristics have not yet been reported and are also not deposited in online databases, the clinical presentation of subject no. 10 is reported here.
The female patient was referred to a local autism center at 3 years of age. Her medical history revealed diagnoses of ASD, global developmental delay, muscle hypotonia, delayed vision maturation, and difficulty walking. A chromosomal microarray was performed at 20 months which was unremarkable, but a whole exome sequence was completed 2 years later revealing the CACNA1D variant. No other genetic abnormalities were found that could explain her symptoms. At the time of presentation to the autism clinic, the patient had been under specialty care from gastroenterology (due to early childhood gastroesophageal reflux and a milk protein allergy), neurology, developmental neurology, developmental pediatrics (medication management of self-harming behaviors), ophthalmology (visual impairment), and orthopedics (for leg/foot misalignment). Despite patient being good-natured and inquisitive, the family was seeking medication management for impulsive and unpredictable self-harming events which occurred multiple times a day including biting herself, scratching to the point of bleeding, throwing herself to the ground, hitting her head against hard surfaces, punching a car so hard it “bloodied her knuckles,” and other significant self-injurious events. Over 2 years of being in the autism clinic, many medications were trialed to provide relief including amantadine, risperidone, memantine, buspirone, quetiapine, gabapentin, and medications targeting underlying anxiety and sleep disorders. Despite periodic improvements with medication management in addition to robust therapeutic services and weekly Applied Behavior Analysis, there was no consistent regimen that controlled her self-harming behaviors and treatment was frequently adjusted. Additionally, a short trial with immediate release isradipine was initiated in an effort to address the underlying calcium channel mutation but was stopped because of concern for worsened symptoms, inefficacy, and complication by a common cold which developed during therapy. During this time, no evidence for cardiovascular abnormalities was found and blood pressure was normal.
From the clinical reports of this and 11 other subjects (Table 3), the following clinically relevant conclusions can be drawn:
-
1.
All affected individuals, except for those with congenital primary aldosteronism (and severe PASNA symptoms, cases no. 1, no. 3, no. 4, and no. 12 in Table 3), have been diagnosed with ASD.
-
2.
Only 2 of the 12 cases (no. 5, no. 6 in Table 3) have been reported with no other neurological or endocrine symptoms in addition to ASD (with or without intellectual impairment) at the time the medical history was published.
-
3.
Endocrine symptoms, driving early genetic diagnosis and immediate therapeutic intervention in the PASNA patients and the individuals affected by congenital hyperinsulinemic hypoglycemia (G403D) or both (L271H, [14]), are observed only in a minority of cases. Their absence does not rule out a CACNA1D channelopathy, and their presence cannot be predicted from the variant gating changes: (i) even variants (such as A749G or S652L) causing almost identical biophysical changes as PASNA variants (I750M) [3, 26, 64, 66, 72] were not associated with clinically overt endocrine symptoms; (ii) an identical variant (G403D) may cause severe disease but with different endocrine manifestations (primary aldosteronism in case no. 1, congenital hyperinsulinemic hypoglycemia in case no. 2); (iii) endocrine symptoms may resolve with age [18, 35].
-
4.
Although endocrine symptoms are not consistently part of the syndrome, individuals with all pathogenic CACNA1D variants should be monitored for symptoms of primary aldosteronism (see case no. 12, [14]) and altered glucose homeostasis even if not present in the peri- and postnatal period. Cav1.3 channels also contribute to the electrical activity and adrenaline secretion in adrenal chromaffin cells in rodents. At present it is unclear if this also results in abnormal adrenaline release in humans. However, measurement of plasma catecholamine levels in affected individuals could address this important question, considering that anxiety is often reported in these patients.
-
5.
Facial dysmorphism or syndactyly, as described in subjects with CACNA1C gain of function variants ([96], see also review in this issue), is not a consistent finding in the CACNA1D cohort (Table 3) but can serve as a supporting diagnostic feature.
-
6.
Autoaggressive and self-harming behaviors have been well-described in the clinical histories of two individuals with different variants (cases no. 7, no. 10) and are considered a behavioral abnormality challenging caregivers and treating physicians.
As described above, Cav1.3 L-type channels are also present in other tissues. Although homozygous loss of Cav1.3 function results in congenital deafness and sinoatrial node dysfunction, it is at present unclear if the heterozygous gain-of-function gating changes of the reported variants result in clinically relevant symptoms on hearing or directly cause the cardiac abnormalities observed in some cases (Table 3).
Repurposing of Ca2+ channel blockers (“Ca2+ antagonists”) for symptom control
As outlined above, the heterozygous de novo CACNA1D variants are dominant in nature and the disease cannot be explained by heterozygous loss of channel function, which is apparently asymptomatic in mice and humans. This is further supported by at least 10 heterozygous protein loss of function variants (stop gained or frameshift located N-terminal to the beginning of the C-terminus) reported in gnomAD (gnomad.broadinstitute.org) samples so far. Based on the enhanced channel activity in heterologous expression systems and promoted Ca2+ signaling in APAs, it is likely that the altered gating changes also increase Cav1.3 channel activity in some populations of neurons. Thus, inhibition of Cav1.3 channel activity with drugs appears as a potential treatment option in affected individuals. Although neurodevelopmental defects are unlikely to be completely reversible, treatment of some otherwise difficult to control symptoms (such as seizures, self-harming behaviors, or muscle hypotonia) may considerably improve the quality of life of patients and their caregivers.
Treatment with Ca2+ channel blockers has already been described in some of the patients, but no conclusive results regarding improvement of neurological or neuropsychiatric symptoms have yet been obtained. The clinical course of the patient described in case no. 1 (G403D, PASNA) was notable for uncontrolled hypertension with hypokalemia (due to primary aldosteronism). Treatment with the dihydropyridine (DHP) Ca2+ channel blocker amlodipine normalized blood pressure and resolved biventricular hypertrophy. Effects of this treatment on other underlying symptoms were not reported [72]. The patient of case no. 2 (same variant) with congenital hyperinsulinemic hypoglycemia received diazoxide (only required until age of 5 years), which successfully controlled blood glucose. Although considered a therapeutic option, treatment with a Ca2+ channel blocker was not reported [18]. As mentioned in the detailed case report above, short treatment of subject no. 10 with the DHP isradipine was also inconclusive. Interestingly, the young subject no. 12 was treated with nifedipine oral solution every 8 h to control hypertension. This not only controlled hypertension but also improved muscle hypotonia. The extent of this improvement was not quantified, and the long-term outcome as well as the tolerability of this treatment was not reported [14]. Nevertheless, it supports the hypothesis that some symptoms may improve upon treatment with DHPs.
What is known about the pharmacology of Cav1.3 that could guide off-label treatment trials in subjects with confirmed pathogenic CACNA1D variants? Both pharmacokinetic and pharmacodynamics need to be considered.
The first question is if Ca2+ channel blockers can sufficiently engage Cav1.3 Ca2+ channels in the brain at plasma levels achieved for antihypertensive therapy. Pharmacokinetic studies in rodents clearly show that some DHPs, such as felodipine and isradipine, used since decades for the treatment of high blood pressure, can quickly and efficiently cross the blood-brain barrier ([76], for review see [43]). An exception seems to be amlodipine. For this widely used DHP, brain exposure after a single dose seems to be lower [88]. Since this compound’s long half-life requires many days of dosing to reach steady state, it is unclear if brain exposure can further increase after multiple dosing.
The second question is if Cav1.3 channels in the brain are efficiently blocked at therapeutic doses of DHPs. The therapeutic target of DHPs for cardiovascular indications is Cav1.2 L-type channels in arterial smooth muscle cells [78, 85]. It is known that under identical experimental conditions Cav1.2 channels are about 5 times more sensitive to inhibition by DHPs than Cav1.3 [62, 94]. This means that higher doses may be required to inhibit Cav1.3 channels in the brain compared with Cav1.2 in the periphery [62]. However, we have recently discovered that some variants can enhance the sensitivity of Cav1.3 channels for inhibition by the DHP isradipine, as shown for S652L [26] and, to a smaller extent, for V401L [65]. This has meanwhile been confirmed also for other variants (NJO, unpublished data). However, we also found the opposite, variants which significantly reduce sensitivity to isradipine (unpublished). This strongly suggests that therapeutic trials should be first started in subjects with DHP-sensitizing variants. If therapy fails in these patients it is unlikely that subjects with other variants would benefit from DHP therapy.
In addition, other factors could be therapy limiting: DHPs are very well tolerated by most patients but may cause hypotension and dizziness at higher doses. Immediate release preparations should be avoided because fast blood pressure lowering may induce sympathetic activation, reflex tachycardia, and flushing, which may cause unwanted behavioral reactions in some patients. Therefore, suitable DHPs require extended release formulations to prevent fast onset, which can be a problem in patients with feeding problems or younger patients, in which oral solutions are more appropriate for administration and correct dosing. In addition, DHPs are cytochrome-P450-3A4 substrates and drug-drug interactions with concomitant therapies (e.g., antiepileptic or psychiatric drugs) have to be considered.
Nevertheless, despite many open questions and potentially therapy-limiting considerations, treatment with Ca2+ channel blockers remains a therapeutic option that should be explored carefully in these patients. These experimental therapies will require not only a skilled therapist but also the patience and support of cooperative parents and their qualified assessment of predefined treatment outcomes.
Somatic CACNA1D APA/APCC variants can help to classify germline variants as high risk
As outlined above, somatic CACNA1D variants in APAs and APCCs cause excess aldosterone production and primary aldosteronism although they seem not to directly contribute to abnormal cell proliferation and adenoma formation [97]. This finding strongly suggests that the gating changes induced by these variants indeed permit enhanced Ca2+ signaling through Cav1.3 channels in these human tumor cells. Accordingly, several variants found in APAs were also reported as pathogenic germline variants (Fig. 2) in a subject with (I750M) and others without endocrine symptoms (S652L, V401L). Therefore, APA/APCC variants could guide the assessment of the potential pathogenicity of new germline variants for which no functional data are available and thus could aid clinical diagnosis. However, a small percentage of APAs and APCCs cannot yet be explained by known somatic de novo mutations [9]. Therefore, it cannot be ruled out completely that a CACNA1D variant is in fact benign and other yet unknown genetic factors account for excess aldosterone production in a given APA/APCC. We therefore propose criteria which classify these somatic variants as pathogenic, likely pathogenic, likely benign or of (yet) uncertain pathogenicity (Table 4). The classified somatic APA/APCC variants as well as their approximate position within the Cav1.3 α1-subunit are given in Table 5 and Supplementary Figure 1, respectively.
Molecular mechanism of altered channel gating in high-risk variants
As mentioned above all germline variants and the vast majority of APA/APCC variants occur in the voltage-sensing or pore-forming domain of the channel (Fig. 2).
We have used the SWISS-MODEL webserver [91] to generate a homology model of CACNA1D based on the apo structure of the inactivated rabbit Cav1.1 α1-subunit in complex with α2δ-, β-, and γ-subunits (PDB entry 5gjv, [93]). Mapping of the mutated residues included in Table 3 onto this structural model revealed spatial clustering around the activation gate of repeats I and II (Fig. 3A). In detail, V401, G403, G407, A749, and I750 are part of the pore-forming S6 segments, whereas V259 and S652 are located in close proximity on neighboring S4-S5 linkers (which connect the voltage-sensing domain (VSD) to the activation gate). These sections of the protein are critical determinants of multiple channel properties: V401 for example is part of a set of hydrophobic residues (V401, F747, F1147, and F1444) lining the pore. These residues form hydrophobic interactions when the pore is closed, thereby prohibiting Ca2+ conductance. Interestingly, variants of two other amino acids of this hydrophobic cluster—F747 and F1147—have been identified in APAs (Table 5). This suggests that disruption of these hydrophobic interactions generally alters channel function, independently of the exact residue affected, and it will be interesting to see whether a variant of the fourth residue in this cluster—F1444—will be observed in patients in the future.
CACNA1D structural models. (A) A homology model of the inactivated state based on rabbit Cav1.1 (PDB entry 5gjv, [93]). Transmembrane segments of homologous repeats I (cyan) and II (blue) are shown with germline de novo missense variants highlighted as orange sticks. They spatially cluster around the activation gate formed by S6-helices of repeats I and II. (B) The homology model of the CACNA1D resting state (based on PDB entry 6p6w, [92]) shows that A749 and G403 (orange spheres) tightly interact with surrounding residues on the S5 helix and S4-S5 linker (highlighted as spheres). Cartoon and carbon atoms depicted as spheres are colored according to the homologous repeats, whereas nitrogen and oxygen atoms of residues depicted as spheres are colored blue and red, respectively. (C) The pore-forming helix S6 requires conformational rearrangements of the backbone to facilitate pore opening in the activated state (green cartoon) compared with the resting state (cyan cartoon). G407 (S6 in repeat I) is highlighted in orange (resting state) and yellow (activated state)
G403 and A749 are part of the S6 G/A/G/A ring found at the intersection of the S4-S5 linker, S5, and S6 helices. They are involved in linker-S6 interactions and have been implicated in coupling of the VSD and the pore [23]. In a homology model of the resting state (based on the resting state of a BacNav channel, PDB entry 6p6w, [92]), these residues fit tightly in the intersection point (Fig. 3B). Therefore, any variant either increasing flexibility and diminishing hydrophobic contacts (i.e., alanine to glycine) or introducing larger and/or polar residues (i.e., alanine to threonine) is expected to interfere with, and thus destabilize, the modeled resting closed state conformation and promote open channel states.
In addition, A749, together with I750, is part of the “LAIA” motif that is highly conserved among L-type Ca2+ channels and mutation of any residues of the motif to proline resulted in altered activation properties in the related Cav1.2 channel [27]. Similar to the G/A/G/A ring, S652 and V259 are within another critical interaction site, connecting the S4-S5 linker of repeats I and II. Previous modeling studies suggested that loss of hydrogen bonds in the S652L variant could be responsible for the effect on channel function [26].
Finally, comparing the resting and activated (based on the active state structure of a BacNav channel, PDB entry 5vb8, [37]) state models of CACNA1D shows that G407 in the S6 segment is located within the pore region, where large movements of the backbone facilitate channel opening and closure (Fig. 3C). Please note that G407 corresponds to the last S6 residue that is still resolved in the template of the BacNav crystal structure, suggesting that downstream residues are subject to conformational flexibility.
Given that structural components regulating both VSD-pore coupling and channel activation as well as pore opening/closure are concentrated in regions surrounding the activation gate, it should not come as a surprise that residues within this area are particularly susceptible to structural changes and give rise to variants that alter the biophysical properties of the channel. We therefore propose that new variants in this region are likely to have a functional impact and should be characterized in detail.
Molecular modeling can also provide evidence for variant R619P as a likely-pathogenic type 4 variant (Table 2). R619W and R619P were reported in APAs and APCCs (Table 5). However, R619W was also reported in a control subject in the gnomAD database (Table 5), casting doubt on its pathogenicity. R619 is the first positive charge in the VSD of repeat II (Fig. 4A). Both the wild-type R619 and the R619W variant provide a steric barrier that seals the VSD and prevents ion leakage through the surface of the VSD (Fig. 4B, D, E). Modeling of the R619P variant suggests that the smaller side chain fails to provide this seal and thus creates a tunnel (Fig. 4C, F). This allows for ion leakage through the VSD in the resting state and thus generates a so-called ω-current, which favors membrane depolarization. Please note that mutation of the uppermost arginine in the VSD has been described as a general mechanism to create gating pores in voltage-gated ion channels [53] and that the Cav1.3 R619P mutant directly corresponds to the Nav1.5 R808P mutant associated with Brugada syndrome (R809P in the rat; [30]). Altogether, this suggests that the Cav1.3 R619P variant also has a high probability of being pathogenic.
Modeling of the R619 variants. (A) R619 (orange sticks and highlighted by dotted lines) is the uppermost positively charged arginine in the VSD of repeat II. For reference, germline variants from Fig. 3A are shown as orange sticks as well. (B + C) Top view from the extracellular side onto the (B) wild-type R (orange sticks), and the W (green sticks) and (C) P (yellow sticks) variants in the Cav1.3 resting state model (gray). (D + E) In the resting state, both R619 (D, orange, highlighted by dotted lines) and the large hydrophobic side chain of the R619W variant (E, green, highlighted by dotted lines) provide a steric barrier that prevents ion leakage through the surface of the VSD (gray surface). (F) The R619P variant (yellow) lacks this large, hydrophobic sidechain, thus creating a tunnel via which ions can potentially pass through to the intracellular side. For comparison, this tunnel is depicted from both the top and bottom view
Estimated prevalence of high-risk CACNA1D de novo variants in neurodevelopmental disorders
Two recently published germline de novo CACNA1D variants identified in small patient cohorts of ASD without (T1376M, [31]) or with epilepsy (V1447L, [44]) could suggest that the prevalence of such de novo CACNA1D variants associated with neurodevelopmental disorders is higher as currently deduced from NGS of large patient cohorts. Both variants are de novo and absent in healthy controls (gnomAD database); however, due to a lack of detailed clinical or functional data, they were not included in Table 3. According to the ACMG classification system [69], we consider the V1447L variant as likely pathogenic due to its location within a well-established functional domain (S6, repeat IV). Importantly, residue V1447 is highly conserved among all Cavs, lies adjacent to the S6 G/A/G/A ring of repeat IV, and corresponds to I750 in repeat II (discussed above), further strengthening the assumption that mutation of this residue can indeed interfere with normal Cav1.3 channel function. This is less clear for the T1376M variant which is located at the beginning of the S5-S6 pore loop of repeat IV, and a functional impact on channel gating cannot be presumed in absence of functional data.
So far only 6 de novo CACNA1D variants have been identified in large-scale genetic studies in cohorts of ASD (A749G, G407R [70], 11,986 individuals; T1376M [31], 59 individuals), ASD with epilepsy (V1447L, [44], 103 individuals), and severe developmental disorders (2 x S652L [15], 1133 individuals). In these cohorts, the occurrence of CACNA1D variants is relatively rare (together 1:2214); thus, they are often not identified as high-risk genetic variants (as happened for S652L in [15] but see [26]). Additionally, patients with pathogenic CACNA1D variants can be affected by endocrine and/or neurological symptoms (Table 3) and might therefore meet the exclusion criteria of such studies, further contributing to their underrepresentation. Since the majority of the known de novo CACNA1D germline variants have been published as case reports or were even unpublished (we are aware of at least two additional unpublished patients affected by one of the pathogenic variants in Table 3), this supports the notion that such CACNA1D variants are likely underreported in the literature.
Summary and perspectives
As described in this review, we begin to understand the clinical disease spectrum associated with pathogenic de novo CACNA1D missense variants. Since the symptoms can only be explained by a channel gain-of-function, L-type Ca2+ channel blockers are a mechanism-based and still promising therapeutic option. However, current data predict that not all variants may be responsive to these drugs and it still remains unclear which of the many neurological and neuropsychiatric symptoms will respond to therapy. To learn more about potential drug effects, it is important to collect information about as many patients as possible which requires that clinicians and geneticists publish newly diagnosed patients as case reports or directly communicate them to members of the scientific community familiar with CACNA1D-associated diseases. But most importantly, all these efforts also depend on the support of parents and/or other caring family members and their willingness to share this information with the scientific community beyond clinical routine. We take this opportunity to thank many of the parents and physicians who helped to provide the clinical information for this review.
Data availability
Not applicable
Change history
14 July 2020
The above article was published online with an error in Article title. Author mispronounced the name of a gene (CACNA1D instead of CACAN1D). The correct gene is presented above.
References
Akerstrom T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, C DV, Backdahl M, Botling J, Stalberg P, Westin G, Walz MK, Lehnert H, Sidhu S, Zedenius J, Bjorklund P, Hellman P (2015) Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer 22:735–744. https://doi.org/10.1530/ERC-15-0321
Azizan EA, Brown MJ (2016) Novel genetic determinants of adrenal aldosterone regulation. Current opinion in endocrinology, diabetes, and obesity 23:209–217. https://doi.org/10.1097/MED.0000000000000255
Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ (2013) Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45:1055–1060. https://doi.org/10.1038/ng.2716
Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nurnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nurnberg P, Striessnig J, Bolz HJ (2011) Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci 14:77–84
Barrett PQ, Guagliardo NA, Klein PM, Hu C, Breault DT, Beenhakker MP (2016) Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol 594:5851–5860. https://doi.org/10.1113/JP271896
Berger SM, Bartsch D (2014) The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res 357:463–476. https://doi.org/10.1007/s00441-014-1936-3
Betzenhauser MJ, Pitt GS, Antzelevitch C (2015) Calcium channel mutations in cardiac arrhythmia syndromes. Curr Mol Pharmacol 8:133–142. https://doi.org/10.2174/1874467208666150518114857
Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A (2011) Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. J Biol Chem 286:42736–42748. https://doi.org/10.1074/jbc.M111.269951
Boulkroun S, Fernandes-Rosa FL, Zennaro MC (2020) Old and new genes in primary aldosteronism. Best practice & research Clinical endocrinology & metabolism:101375. doi:https://doi.org/10.1016/j.beem.2020.101375
Brandt A, Striessnig J, Moser T (2003) CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci 23:10832–10840. https://doi.org/10.1523/JNEUROSCI.23-34-10832.2003
Busquet P, Nguyen NK, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N (2010) Cav1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int J Neuropsychopharmacol 13:499–513. https://doi.org/10.1017/S1461145709990368
Cannon SC (2010) Voltage-sensor mutations in channelopathies of skeletal muscle. J Physiol 588:1887–1895. https://doi.org/10.1113/jphysiol.2010.186874
Chemin J, Siquier-Pernet K, Nicouleau M, Barcia G, Ahmad A, Medina-Cano D, Hanein S, Altin N, Hubert L, Bole-Feysot C, Fourage C, Nitschke P, Thevenon J, Rio M, Blanc P, Vidal C, Bahi-Buisson N, Desguerre I, Munnich A, Lyonnet S, Boddaert N, Fassi E, Shinawi M, Zimmerman H, Amiel J, Faivre L, Colleaux L, Lory P, Cantagrel V (2018) De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain 141:1998–2013. https://doi.org/10.1093/brain/awy145
De Mingo Alemany MC, Mifsud Grau L, Moreno Macian F, Ferrer Lorente B, Leon Carinena S (2020) A de novo Cacna1d missense mutation in a patient with congenital hyperinsulinism. Primary Hyperaldosteronism and Hypotonia Channels (Austin) 14:175–180. https://doi.org/10.1080/19336950.2020.1761171
Deciphering Developmental Disorders S (2015) Large-scale discovery of novel genetic causes of developmental disorders. Nature 519:223–228. https://doi.org/10.1038/nature14135
Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi K, Aritomi S, Ikeda K, Yoshimura M, Tojo K, Sasano H (2014) Voltage-gated calcium channels in the human adrenal and primary aldosteronism. J Steroid Biochem Mol Biol 144 Pt B:410-416. https://doi.org/10.1016/j.jsbmb.2014.08.012
Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC (2014) Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 64:354–361. https://doi.org/10.1161/HYPERTENSIONAHA.114.03419
Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, Hussain K, Ellard S (2017) A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes 18:320–323. https://doi.org/10.1111/pedi.12512
Garza-Lopez E, Lopez JA, Hagen J, Sheffer R, Meiner V, Lee A (2018) Role of a conserved glutamine in the function of voltage-gated Ca(2+) channels revealed by a mutation in human CACNA1D. J Biol Chem 293:14444–14454. https://doi.org/10.1074/jbc.RA118.003681
Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE, Rajadhyaksha AM (2010) Molecular switch from L-type Ca v 1.3 to Ca v 1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci 30:17051–17062. https://doi.org/10.1523/JNEUROSCI.2255-10.2010
Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT, Surmeier DJ (2018) Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J Clin Invest 128:2266–2280. https://doi.org/10.1172/JCI95898
Helbig KL, Lauerer RJ, Bahr JC, Souza IA, Myers CT, Uysal B, Schwarz N, Gandini MA, Huang S, Keren B, Mignot C, Afenjar A, Billette de Villemeur T, Heron D, Nava C, Valence S, Buratti J, Fagerberg CR, Soerensen KP, Kibaek M, Kamsteeg EJ, Koolen DA, Gunning B, Schelhaas HJ, Kruer MC, Fox J, Bakhtiari S, Jarrar R, Padilla-Lopez S, Lindstrom K, Jin SC, Zeng X, Bilguvar K, Papavasileiou A, Xin Q, Zhu C, Boysen K, Vairo F, Lanpher BC, Klee EW, Tillema JM, Payne ET, Cousin MA, Kruisselbrink TM, Wick MJ, Baker J, Haan E, Smith N, Corbett MA, MacLennan AH, Gecz J, Biskup S, Goldmann E, Rodan LH, Kichula E, Segal E, Jackson KE, Asamoah A, Dimmock D, McCarrier J, Botto LD, Filloux F, Tvrdik T, Cascino GD, Klingerman S, Neumann C, Wang R, Jacobsen JC, Nolan MA, Snell RG, Lehnert K, Sadleir LG, Anderlid BM, Kvarnung M, Guerrini R, Friez MJ, Lyons MJ, Leonhard J, Kringlen G, Casas K, El Achkar CM, Smith LA, Rotenberg A, Poduri A, Sanchis-Juan A, Carss KJ, Rankin J, Zeman A, Raymond FL, Blyth M, Kerr B, Ruiz K, Urquhart J, Hughes I, Banka S, Deciphering Developmental Disorders S, Hedrich UBS, Scheffer IE, Helbig I, Zamponi GW, Lerche H, Mefford HC (2018) De novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and dyskinesias. Am J Hum Genet 103:666–678. https://doi.org/10.1016/j.ajhg.2018.09.006
Hering S, Zangerl-Plessl EM, Beyl S, Hohaus A, Andranovits S, Timin EN (2018) Calcium channel gating. Pflugers Arch 470:1291–1309. https://doi.org/10.1007/s00424-018-2163-7
Hirtz JJ, Boesen M, Braun N, Deitmer JW, Kramer F, Lohr C, Muller B, Nothwang HG, Striessnig J, Lohrke S, Friauf E (2011) Cav1.3 calcium channels are required for normal development of the auditory brainstem. J Neurosci 31:8280–8294
Hirtz JJ, Braun N, Griesemer D, Hannes C, Janz K, Lohrke S, Muller B, Friauf E (2012) Synaptic refinement of an inhibitory topographic map in the auditory brainstem requires functional Cav1.3 calcium channels. J Neurosci 32:14602–14616. https://doi.org/10.1523/JNEUROSCI.0765-12.2012
Hofer NT, Tuluc P, Ortner NJ, Nikonishyna YV, Fernandes-Quintero ML, Liedl KR, Flucher BE, Cox H, Striessnig J (2020) Biophysical classification of a CACNA1D de novo mutation as a high-risk mutation for a severe neurodevelopmental disorder. Mol Autism 11:4. https://doi.org/10.1186/s13229-019-0310-4
Hohaus A, Beyl S, Kudrnac M, Berjukow S, Timin EN, Marksteiner R, Maw MA, Hering S (2005) Structural determinants of L-type channel activation in segment IIS6 revealed by a retinal disorder. J Biol Chem 280:38471–38477. https://doi.org/10.1074/jbc.M507013200
Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299. https://doi.org/10.1016/j.neuron.2012.04.009
Jiang D, Gamal El-Din TM, Ing C, Lu P, Pomes R, Zheng N, Catterall WA (2018) Structural basis for gating pore current in periodic paralysis. Nature 557:590–594. https://doi.org/10.1038/s41586-018-0120-4
Jiang D, Shi H, Tonggu L, Gamal El-Din TM, Lenaeus MJ, Zhao Y, Yoshioka C, Zheng N, Catterall WA (2020) Structure of the cardiac sodium channel. Cell 180:122–134.e10. https://doi.org/10.1016/j.cell.2019.11.041
Jiao J, Zhang M, Yang P, Huang Y, Hu X, Cai J, Yang C, Situ M, Zhang H, Fu L, Guo K, Huang Y (2020) Identification of de novo JAK2 and MAPK7 mutations related to autism spectrum disorder using whole-exome sequencing in a Chinese child and adolescent trio-based sample. J Mol Neurosci 70:219–229. https://doi.org/10.1007/s12031-019-01456-z
Jurkat-Rott K, Groome J, Lehmann-Horn F (2012) Pathophysiological role of omega pore current in channelopathies. Front Pharmacol 3:112. https://doi.org/10.3389/fphar.2012.00112
Kabir ZD, Lee AS, Rajadhyaksha AM (2016) L-type Ca channels in mood, cognition and addiction: integrating human and rodent studies with a focus on behavioural endophenotypes. J Physiol 594:5823–5837. https://doi.org/10.1113/JP270673
Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T (2016) Clinical and Steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab 101:494–503. https://doi.org/10.1210/jc.2015-3284
Korah HE, Scholl UI (2015) An update on familial hyperaldosteronism. Horm Metab Res 47:941–946. https://doi.org/10.1055/s-0035-1564166
Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, Rajadhyaksha AM (2012) Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry 17:1054–1055. https://doi.org/10.1038/mp.2012.71
Lenaeus MJ, Gamal El-Din TM, Ing C, Ramanadane K, Pomes R, Zheng N, Catterall WA (2017) Structures of closed and open states of a voltage-gated sodium channel. Proc Natl Acad Sci U S A 114:E3051–E3060. https://doi.org/10.1073/pnas.1700761114
Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, Tsimberidou AM, Vnencak-Jones CL, Wolff DJ, Younes A, Nikiforova MN (2017) Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 19:4–23. https://doi.org/10.1016/j.jmoldx.2016.10.002
Liao P, Soong TW (2010) CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch 460:353–359. https://doi.org/10.1007/s00424-009-0753-0
Lieb A, Ortner N, Striessnig J (2014) C-terminal modulatory domain controls coupling of voltage-sensing to pore opening in Cav1.3 L-type Ca(2+) channels. Biophys J 106:1467–1475. https://doi.org/10.1016/j.bpj.2014.02.017
Limpitikul WB, Dick IE, Ben-Johny M, Yue DT (2016) An autism-associated mutation in CaV1.3 channels has opposing effects on voltage- and Ca(2+)-dependent regulation. Sci Rep 6:27235. doi:https://doi.org/10.1038/srep27235
Lipscombe D, Andrade A, Allen SE (2012) Alternative splicing: functional diversity among voltage-gated calcium channels and behavioral consequences. Biochim Biophys Acta
Liss B, Striessnig J (2019) The potential of L-type calcium channels as a drug target for neuroprotective therapy in Parkinson’s disease. Annu Rev Pharmacol Toxicol 59:263–289. https://doi.org/10.1146/annurev-pharmtox-010818-021214
Long S, Zhou H, Li S, Wang T, Ma Y, Li C, Zhou Y, Zhou S, Wu B, Wang Y (2019) The clinical and genetic features of co-occurring epilepsy and autism spectrum disorder in Chinese children. Front Neurol 10:505. https://doi.org/10.3389/fneur.2019.00505
Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J (2003) Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A 100:5543–5548. https://doi.org/10.1073/pnas.0935295100
Marcantoni A, Vandael DH, Mahapatra S, Carabelli V, Sinnegger-Brauns MJ, Striessnig J, Carbone E (2010) Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci 30:491–504
Marger L, Mesirca P, Alig J, Torrente A, Dubel S, Engeland B, Kanani S, Fontanaud P, Striessnig J, Shin HS, Isbrandt D, Ehmke H, Nargeot J, Mangoni ME (2011) Functional roles of Ca(v)1.3, Ca(v)3.1 and HCN channels in automaticity of mouse atrioventricular cells: insights into the atrioventricular pacemaker mechanism. Channels (Austin) 5:251–261
Marschallinger J, Sah A, Schmuckermair C, Unger M, Rotheneichner P, Kharitonova M, Waclawiczek A, Gerner P, Jaksch-Bogensperger H, Berger S, Striessnig J, Singewald N, Couillard-Despres S, Aigner L (2015) The L-type calcium channel Cav1.3 is required for proper hippocampal neurogenesis and cognitive functions. Cell Calcium 58:606–616. https://doi.org/10.1016/j.ceca.2015.09.007
Martinez-Rivera A, Hao J, Tropea TF, Giordano TP, Kosovsky M, Rice RC, Lee A, Huganir RL, Striessnig J, Addy NA, Han S, Rajadhyaksha AM (2017) Enhancing VTA Cav1.3 L-type Ca(2+) channel activity promotes cocaine and mood-related behaviors via overlapping AMPA receptor mechanisms in the nucleus accumbens. Mol Psychiatry 22:1735–1745. https://doi.org/10.1038/mp.2017.9
McKinney BC, Murphy GG (2006) The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem 13:584–589. https://doi.org/10.1101/lm.279006
McKinney BC, Sze W, Lee B, Murphy GG (2009) Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem 92:519–528. https://doi.org/10.1016/j.nlm.2009.06.012
Monteleone S, Lieb A, Pinggera A, Negro G, Fuchs JE, Hofer F, Striessnig J, Tuluc P, Liedl KR (2017) Mechanisms responsible for omega-pore currents in Cav calcium channel voltage-sensing domains. Biophys J 113:1485–1495. https://doi.org/10.1016/j.bpj.2017.08.010
Moreau A, Chahine M (2018) A new cardiac channelopathy: from clinical phenotypes to molecular mechanisms associated with Nav1.5 gating pores. Frontiers in cardiovascular medicine 5:139. doi:https://doi.org/10.3389/fcvm.2018.00139
Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS (2001) Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest 108:1015–1022. https://doi.org/10.1172/JCI13310
Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE (2016) Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab 101:999–1007. https://doi.org/10.1210/jc.2015-3239
Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, Vaidya A, Barletta JA, Else T, Giordano TJ, Tomlins SA, Rainey WE (2019) Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension 73:885–892. https://doi.org/10.1161/HYPERTENSIONAHA.118.12070
Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE (2015) Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 112:E4591–E4599. https://doi.org/10.1073/pnas.1505529112
O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250. https://doi.org/10.1038/nature10989
Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, Ito S, Satoh F, Sasano H, Rainey WE, Tomlins SA (2017) Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in Normal adrenals. Journal of the Endocrine Society 1:787–799. https://doi.org/10.1210/js.2017-00134
Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand SK, Guo Z, Stowasser M, Sasano H, Tomlins SA, Rainey WE (2018) Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension 72:874–880. https://doi.org/10.1161/HYPERTENSIONAHA.118.11086
Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87:543–552. https://doi.org/10.1016/s0092-8674(00)81373-2
Ortner NJ, Bock G, Dougalis A, Kharitonova M, Duda J, Hess S, Tuluc P, Pomberger T, Stefanova N, Pitterl F, Ciossek T, Oberacher H, Draheim HJ, Kloppenburg P, Liss B, Striessnig J (2017) Lower affinity of isradipine for L-type Ca2+ channels during substantia nigra dopamine neuron-like activity: implications for neuroprotection in Parkinson’s disease. J Neurosci 37:6761–6777
Pietrobon D (2010) CaV2.1 channelopathies. Pflugers Arch 460:375–393. https://doi.org/10.1007/s00424-010-0802-8
Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J (2015) CACNA1D De novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry 77:816–822. https://doi.org/10.1016/j.biopsych.2014.11.020
Pinggera A, Mackenroth L, Rump A, Schallner J, Beleggia F, Wollnik B, Striessnig J (2017) New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum Mol Genet 26:2923–2932. https://doi.org/10.1093/hmg/ddx175
Pinggera A, Negro G, Tuluc P, Brown MJ, Lieb A, Striessnig J (2018) Gating defects of disease-causing de novo mutations in Cav1.3 Ca(2+) channels. Channels (Austin) 12:388–402. https://doi.org/10.1080/19336950.2018.1546518
Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J (2000) Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102:89–97
Reinbothe TM, Alkayyali S, Ahlqvist E, Tuomi T, Isomaa B, Lyssenko V, Renstrom E (2013) The human L-type calcium channel Cav1.3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia 56:340–349. https://doi.org/10.1007/s00125-012-2758-z
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, Stevens C, Reichert J, Mulhern MS, Artomov M, Gerges S, Sheppard B, Xu X, Bhaduri A, Norman U, Brand H, Schwartz G, Nguyen R, Guerrero EE, Dias C, Autism Sequencing C, i P-BC, Betancur C, Cook EH, Gallagher L, Gill M, Sutcliffe JS, Thurm A, Zwick ME, Borglum AD, State MW, Cicek AE, Talkowski ME, Cutler DJ, Devlin B, Sanders SJ, Roeder K, Daly MJ, Buxbaum JD (2020) Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180:568–584.e23. https://doi.org/10.1016/j.cell.2019.12.036
Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM (2011) Cav1.2 L-type Ca(2)(+) channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. J Neurosci 31:13562–13575. https://doi.org/10.1523/JNEUROSCI.2315-11.2011
Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP (2013) Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45:1050–1054. https://doi.org/10.1038/ng.2695
Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kucukkoylu S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T (2015) Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol 83:779–789. https://doi.org/10.1111/cen.12873
Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP (2015) Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 4:e06315. https://doi.org/10.7554/eLife.06315
Semenova NA, Ryzhkova OR, Strokova TV, Taran NN (2018) The third case report a patient with primary aldosteronism, seizures, and neurologic abnormalities (PASNA) syndrome de novo variant mutations in the CACNA1D gene. Zh Nevrol Psikhiatr Im S S Korsakova 118:49–52. https://doi.org/10.17116/jnevro201811812149
Siddiqi FH, Menzies FM, Lopez A, Stamatakou E, Karabiyik C, Ureshino R, Ricketts T, Jimenez-Sanchez M, Esteban MA, Lai L, Tortorella MD, Luo Z, Liu H, Metzakopian E, Fernandes HJR, Bassett A, Karran E, Miller BL, Fleming A, Rubinsztein DC (2019) Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat Commun 10:1817. https://doi.org/10.1038/s41467-019-09494-2
Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A (2008) Modulation of voltage- and Ca2+-dependent gating of Cav1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem 283:20733–20744. https://doi.org/10.1074/jbc.M802254200
Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschutz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig J (2004) Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest 113:1430–1439. https://doi.org/10.1172/JCI20208
Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J (2009) Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 75:407–414. https://doi.org/10.1124/mol.108.049981
Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT (2005) Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A 102:8089–8096; discussion 8086-8088. https://doi.org/10.1073/pnas.0502506102
Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT (2004) Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119:19–31. https://doi.org/10.1016/j.cell.2004.09.011
Strauss KA, Gonzaga-Jauregui C, Brigatti KW, Williams KB, King AK, Van Hout C, Robinson DL, Young M, Praveen K, Heaps AD, Kuebler M, Baras A, Reid JG, Overton JD, Dewey FE, Jinks RN, Finnegan I, Mellis SJ, Shuldiner AR, Puffenberger EG (2018) Genomic diagnostics within a medically underserved population: efficacy and implications. Genet Med 20:31–41. https://doi.org/10.1038/gim.2017.76
Striessnig J (2013) Lonely but diverse: Cav1.3 L-type Ca ( 2+) channels in cochlear inner hair cells. Channels (Austin) 7:133–134. https://doi.org/10.4161/chan.24457
Striessnig J, Bolz HJ, Koschak A (2010) Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch 460:361–374. https://doi.org/10.1007/s00424-010-0800-x
Striessnig J, Ortner NJ (2020) Ca2+ channel blockers. In: Offermanns S, Rosenthal W (eds) Encyclopedia of Molecular Pharmacology Springer, Cham
Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet 19:260–263. https://doi.org/10.1038/940
Tan GC, Negro G, Pinggera A, Tizen Laim NMS, Mohamed Rose I, Ceral J, Ryska A, Chin LK, Kamaruddin NA, Mohd Mokhtar N, AR AJ, Sukor N, Solar M, Striessnig J, Brown MJ, Azizan EA (2017) Aldosterone-producing adenomas: histopathology-genotype correlation and identification of a novel CACNA1D mutation. Hypertension 70:129–136. https://doi.org/10.1161/HYPERTENSIONAHA.117.09057
Uchida S, Yamada S, Nagai K, Deguchi Y, Kimura R (1997) Brain pharmacokinetics and in vivo receptor binding of 1,4-dihydropyridine calcium channel antagonists. Life Sci 61:2083–2090
Vandael DH, Marcantoni A, Carbone E (2015) Cav1.3 channels as key regulators of neuron-like firings and catecholamine release in chromaffin cells. Curr Mol Pharmacol 8:149–161
Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, Fan Y, Ouyang J (2015) Prevalence and characterization of somatic mutations in chinese aldosterone-producing adenoma patients. Medicine (Baltimore) 94:e708. https://doi.org/10.1097/MD.0000000000000708
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Wisedchaisri G, Tonggu L, McCord E, Gamal El-Din TM, Wang L, Zheng N, Catterall WA (2019) Resting-state structure and gating mechanism of a voltage-gated sodium channel. Cell 178:993–1003.e12. https://doi.org/10.1016/j.cell.2019.06.031
Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q, Yan N (2016) Structure of the voltage-gated calcium channel Cav1.1 at 3.6 A resolution. Nature 537:191–196. https://doi.org/10.1038/nature19321
Xu W, Lipscombe D (2001) Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21:5944–5951. https://doi.org/10.1523/JNEUROSCI.21-16-05944.2001
Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, Rainey WE, Ito S, Satoh F, Sasano H (2017) Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab 102:1182–1192. https://doi.org/10.1210/jc.2016-2986
Zamponi GW, Striessnig J, Koschak A, Dolphin AC (2015) The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 67:821–870. https://doi.org/10.1124/pr.114.009654
Zennaro MC, Boulkroun S, Fernandes-Rosa F (2017) Genetic causes of functional adrenocortical adenomas. Endocr Rev 38:516–537. https://doi.org/10.1210/er.2017-00189
Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N (2002) Functional roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res 90:981–987
Zhao Y, Huang G, Wu Q, Wu K, Li R, Lei J, Pan X, Yan N (2019) Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature 576:492–497. https://doi.org/10.1038/s41586-019-1801-3
Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, Wang JG, Zhu DL, Gao PJ (2015) Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 65:622–628. https://doi.org/10.1161/HYPERTENSIONAHA.114.03346
Acknowledgments
Open access funding provided by Austrian Science Fund (FWF).
Code availability
Not applicable
Funding
Austrian Science Fund (FWF, P27809 and DOC30/CavX).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Channelopathies: from mutation to diseases in Pflügers Archiv—European Journal of Physiology
Electronic supplementary material
Supplementary Fig. 1
Somatic de novoCACNA1Dvariants found in APAs and APCCs. The predicted pathogenicity of the variants (for details see Tables 4 and 5) is indicated by the following color code: Pathogenic (red), likely pathogenic (blue), likely benign (green), uncertain (orange). (JPG 265 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ortner, N.J., Kaserer, T., Copeland, J.N. et al. De novo CACAN1D Ca2+ channelopathies: clinical phenotypes and molecular mechanism. Pflugers Arch - Eur J Physiol 472, 755–773 (2020). https://doi.org/10.1007/s00424-020-02418-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02418-w