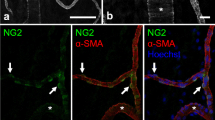
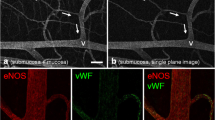
Abstract
Mural cells in precapillary arterioles (PCAs) generate spontaneous Ca2+ transients primarily arising from the periodic release of Ca2+ from sarcoendoplasmic reticulum (SR/ER). The Ca2+ release induces Ca2+-activated chloride channel (CaCC)-dependent depolarisations that spread to neighbouring mural cells to develop the synchrony of their Ca2+ transients. Here, we explored the roles of K+ channels in maintaining the synchrony of spontaneous Ca2+ transients. Intracellular Ca2+ dynamics in mural cells were visualised by Cal-520 fluorescence Ca2+ imaging in the submucosal PCAs of rat rectum. Increasing extracellular K+ concentration ([K+]o) from 5.9 to 29.7 mM converted synchronous spontaneous Ca2+ transients into asynchronous, high-frequency Ca2+ transients. Similarly, the blockade of inward rectifier K+ (Kir) channels with Ba2+ (50 μM) or Kv7 voltage-dependent K+ (Kv7) channels with XE 991 (10 μM) disrupted the synchrony of spontaneous Ca2+ transients, while the blockers for large-, intermediate- or small-conductance Ca2+-activated K+ channels had no effect. Kir2.1 immunoreactivity was detected in the arteriolar endothelium but not mural cells. In the PCAs that had been pretreated with XE 991 or Ba2+, nifedipine (1 μM) attenuated the asynchronous Ca2+ transients but failed to restore their synchrony. In contrast, levcromakalim, an ATP-sensitive K+ channel opener, restored the synchronous Ca2+ transients. Thus, constitutively active Kv7 and Kir channels appear to be involved in maintaining the relatively hyperpolarised membrane of mural cells. The hyperpolarised membrane prevents depolarisation-induced ‘premature’ Ca2+ transients to ensure sufficient SR/ER Ca2+ refilling that is required for regenerative Ca2+ release resulting in synchronous Ca2+ transients amongst the mural cells.









Similar content being viewed by others
Abbreviations
- AUC:
-
Area under curve
- CaCC:
-
Ca2+-activated Cl− channel
- cGMP:
-
Cyclic guanosine monophosphate
- eNOS:
-
Endothelial nitric oxide synthase
- IK channel:
-
Intermediate-conductance Ca2+-activated K+ channel
- [K+]o :
-
Extracellular K+ concentration
- KATP channel:
-
ATP-sensitive K+ channel
- Kir channel:
-
Inward rectifier K+ channel
- Kv7 channel:
-
Kv7 voltage-dependent K+ channel
- LVDCC:
-
L-type voltage-dependent Ca2+ channel
- NO:
-
Nitric oxide
- PCA:
-
Precapillary arteriole
- PSS:
-
Physiological salt solution
- SK channel:
-
Small-conductance Ca2+-activated K+ channel
- SR/ER:
-
Sarcoendoplasmic reticulum
- TTX:
-
Tetrodotoxin
- TVDCC:
-
T-type voltage-dependent Ca2+ channel
References
Anderson UA, Carson C, Johnston L, Joshi S, Gurney AM, McCloskey KD (2013) Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br J Pharmacol 169:1290–1304
Balemba OB, Bartoo AC, Nelson MT, Mawe GM (2008) Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol 294:G467–G476
Berridge MJ, Galione A (1988) Cytosolic calcium oscillators. FASEB J 2:3074–3082
Best L, Bolton TB (1986) Depolarisation of guinea-pig visceral smooth muscle causes hydrolysis of inositol phospholipids. Naunyn Schmiedeberg's Arch Pharmacol 333:78–82
Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW (2000) Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407:870–876
Cao C, Lee-Kwon W, Silldorff EP, Pallone TL (2005) KATP channel conductance of descending vasa recta pericytes. Am J Physiol Ren Physiol 289:F1235–F1245
Cao C, Goo JH, Lee-Kwon W, Pallone TL (2006) Vasa recta pericytes express a strong inward rectifier K+ conductance. Am J Physiol Regul Integr Comp Physiol 290:R1601–R1607
Chen G, Suzuki H, Weston AH (1988) Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95:1165–1174
Colantuoni A, Bertuglia S, Intaglietta M (1984) Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Phys 246:H508–H517
Crane GJ, Gallagher N, Dora KA, Garland CJ (2003) Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553:183–189
Dongaonkar RM, Quick CM, Vo JC, Meisner JK, Laine GA, Davis MJ, Stewart RH (2012) Blood flow augmentation by intrinsic venular contraction in vivo. Am J Physiol Regul Integr Comp Physiol 302:R1436–R1442
Dopico AM, Bukiya AN, Jaggar JH (2018) Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch 470:1271–1289
Edwards FR, Hirst GD (1988) Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol 404:437–454
Fukuta H, Kito Y, Suzuki H (2002) Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle. J Physiol 540:249–260
Haddock RE, Hill CE (2005) Rhythmicity in arterial smooth muscle. J Physiol 566:645–656
Hashitani H, Lang RJ (2016) Spontaneous activity in the microvasculature of visceral organs: role of pericytes and voltage-dependent Ca2+ channels. J Physiol 594:555–565
Hashitani H, Mitsui R (2019) Role of pericytes in the initiation and propagation of spontaneous activity in the microvasculature. In: Smooth muscle spontaneous activity—physiological and pathological modulation. Springer. https://doi.org/10.1007/978-981-13-5895-1
Hashitani H, Suzuki H (1997) K+ channels which contribute to the acetylcholine-induced hyperpolarization in smooth muscle of the guinea-pig submucosal arteriole. J Physiol 501:319–329
Hashitani H, Windle A, Suzuki H (1998) Neuroeffector transmission in arterioles of the guinea-pig choroid. J Physiol 510:209–223
Hashitani H, Lang RJ, Mitsui R, Mabuchi Y, Suzuki H (2009) Distinct effects of CGRP on typical and atypical smooth muscle cells involved in generating spontaneous contractions in the mouse renal pelvis. Br J Pharmacol 158:2030–2045
Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H (2011) Functional properties of suburothelial microvessels in the rat bladder. J Urol 185:2382–2391
Hashitani H, Mitsui R, Masaki S, van Helden DF (2015) Pacemaker role of pericytes in generating synchronised spontaneous Ca2+ transients in the myenteric microvasculature of the guinea-pig gastric antrum. Cell Calcium 58:442–456
Hashitani H, Mitsui R, Miwa-Nishimura K, Lam M (2018) Role of capillary pericytes in the integration of spontaneous Ca2+ transients in the suburothelial microvasculature in situ of the mouse bladder. J Physiol 596:3531–3552
Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J (2015) Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87:95–110
Hundley WG, Renaldo GJ, Levasseur JE, Kontos HA (1988) Vasomotion in cerebral microcirculation of awake rabbits. Am J Phys 254:H67–H71
Imtiaz MS, Smith DW, van Helden DF (2002) A theoretical model of slow wave regulation using voltage-dependent synthesis of inositol 1,4,5-trisphosphate. Biophys J 83:1877–1890
Ishizaki E, Fukumoto M, Puro DG (2009) Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol 587:2233–2253
Jones TW (1852) Discovery that the veins of the bat's wing (which are furnished with valves) are endowed with rhythmical contractility, and that the onward flow of blood is accelerated by each contraction. Philos Trans R Soc Lond 142:131–136
Komori K, Lorenz RR, Vanhoutte PM (1988) Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Phys 255:H207–H212
Lang RJ, Hashitani H, Tonta MA, Parkington HC, Suzuki H (2007) Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol 583:1049–1068
Lee K, Isogai A, Antoh M, Kajioka S, Eto M, Hashitani H (2018) Role of K+ channels in regulating spontaneous activity in the muscularis mucosae of guinea pig bladder. Eur J Pharmacol 818:30–37
Longden TA, Nelson MT (2015) Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22:183–196
Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT (2017) Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20:717–726
Matsushita K, Puro DG (2006) Topographical heterogeneity of KIR currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol 573:483–495
Mauban JR, Wier WG (2004) Essential role of EDHF in the initiation and maintenance of adrenergic vasomotion in rat mesenteric arteries. Am J Physiol Heart Circ Physiol 287:H608–H616
Mitsui R, Hashitani H (2015) Functional properties of submucosal venules in the rat stomach. Pflugers Arch 467:1327–1342
Mitsui R, Hashitani H (2016) Mechanisms underlying spontaneous constrictions of postcapillary venules in the rat stomach. Pflugers Arch 468:279–291
Mitsui R, Hashitani H (2017) Properties of synchronous spontaneous Ca2+ transients in the mural cells of rat rectal arterioles. Pflugers Arch 469:1189–1202
Mitsui R, Miyamoto S, Takano H, Hashitani H (2013) Properties of submucosal venules in the rat distal colon. Br J Pharmacol 170:968–977
Nelson MT, Patlak JB, Worley JF, Standen NB (1990) Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Phys 259:C3–C18
O'Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D (2017) Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 6:e29280
Okazaki K, Seki S, Kanaya N, Hattori J, Tohse N, Namiki A (2003) Role of endothelium-derived hyperpolarizing factor in phenylephrine-induced oscillatory vasomotion in rat small mesenteric artery. Anesthesiology 98:1164–1171s
Sergeant GP, Bradley E, Thornbury KD, McHale NG, Hollywood MA (2008) Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol 586:4631–4642
Shimizu Y, Mochizuki S, Mitsui R, Hashitani H (2014) Neurohumoral regulation of spontaneous constrictions in suburothelial venules of the rat urinary bladder. Vasc Pharmacol 60:84–94
Slaaf DW, Tangelder GJ, Teirlinck HC, Reneman RS (1987) Arteriolar vasomotion and arterial pressure reduction in rabbit tenuissimus muscle. Microvasc Res 33:71–80
Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM (2000) Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol 525:355–361
Yeung S, Schwake M, Pucovský V, Greenwood IA (2008) Bimodal effects of the Kv7 channel activator retigabine on vascular K+ currents. Br J Pharmacol 155:62–72
Acknowledgments
The authors wish to thank Dr. Richard Lang (Monash University) for his critical reading of the manuscript and also Dr. Hiromichi Takano (Nagoya City University) for his advice on experiments.
Funding
The present study was partly supported by Grant-in-Aid for Young Scientists (B) (No. 16K19361) from Japan Society for Promotion of the Science (JSPS) to R.M., Grant-in-Aid for Scientific Research (C) (No. 17K11187) from JSPS to H.H. and a grant-in-aid of the 24th General Assembly of the Japanese Association of Medical Sciences to R.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethics
The experimental protocols in the present study were approved by the animal experimentation ethics committee at Nagoya City University Graduate School of Medical Sciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitsui, R., Hashitani, H. Role of K+ channels in maintaining the synchrony of spontaneous Ca2+ transients in the mural cells of rat rectal submucosal arterioles. Pflugers Arch - Eur J Physiol 471, 1025–1040 (2019). https://doi.org/10.1007/s00424-019-02274-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02274-3