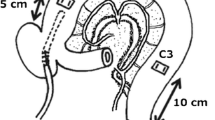
Abstract
Nutrients in the lumen of the small intestine are sensed by special cells in the epithelial lining. The ensuing neurohumoral reflexes affect gastrointestinal absorption/secretion, motility, and vascular perfusion. To study in vivo the effect of a monosaccharide (glucose) or polysaccharide (starch) present in the jejunum on glucose absorption from an adjacent part of the intestine and investigate the possible underlying mechanisms. Using the single pass intraluminal perfusion technique, a segment of jejunum (perfusion segment) was continuously perfused with 20 mM glucose to determine glucose absorption. One hour later, a bolus of a saccharide was instilled in an isolated adjacent jejunal segment and the change in glucose absorption was monitored for a further 2 h. The contribution of neural mechanisms in this process was investigated. Instillation of glucose (20 mM or 40 mM) in either distal or proximal jejunal pouch elicited immediate and sustained inhibition of glucose absorption (a decrease by 25%; P < 0.01) from the perfused jejunal segment. Comparable inhibition was obtained with instillation of other monosaccharides or starch in the jejunal pouch. This inhibition was abolished by adding tetrodotoxin to the pouch or to the perfused jejunal segment and also by pretreatment with sympathetic blockers (guanethidine or hexamethonium) and by chemical ablation of capsaicin-sensitive primary afferent fibers. Glucose absorption within the jejunum is auto-regulated through backward and forward mechanisms. This regulation is mediated by neural reflexes involving capsaicin-sensitive afferent and sympathetic efferent fibers. These reflexes might serve to protect against hyperglycemia.







Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Barada KA, Mourad FH, Bou Raad R, Khoury C, Saade NE, Nassar CF (2008) Neural regulation of glucose absorption in rat jejunum: dual role of capsaicin sensitive primary afferents. Gut 57(suppl 2):A221
Barada KA, Saade NE, Atweh SF, Nassar CF (1998) Neural mediation of vasoactive intestinal polypeptide inhibitory effect on jejunal alanine absorption. Am J Phys 275:G822–G828
Bates SL, Sharkey KA, Meddings JB (1998) Vagal involvement in dietary regulation of nutrient transport. Am J Phys 274:G552–G560
Bierhoff ML, Levine GM (1988) Luminal and metabolic regulation of jejunal amino acid absorption in the rat. Gastroenterology 95:63–68
Boron WF, Boulpaep EL (1989) The electrogenic Na/HCO3 cotransporter. Kidney Int 36:392–402
Buchan AM (1999) Nutrient tasting and signaling mechanisms in the gut III. Endocrine cell recognition of luminal nutrients. Am J Phys 277:G1103–G1107
Debnam ES (1985) Adaptation of hexose uptake by the rat jejunum induced by the perfusion of sugars into the distal ileum. Digestion 31:25–30
Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H (2003) A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A 100:11753–11758. https://doi.org/10.1073/pnas.1733027100
Drozdowski LA, Thomson AB (2006) Intestinal sugar transport. World J Gastroenterol 12:1657–1670
Dyer J, Daly K, Salmon KS, Arora DK, Kokrashvili Z, Margolskee RF, Shirazi-Beechey SP (2007) Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans 35:1191–1194. https://doi.org/10.1042/BST0351191
Dyer J, Hosie KB, Shirazi-Beechey SP (1997) Nutrient regulation of human intestinal sugar transporter (SGLT1) expression. Gut 41:56–59
Dyer J, Vayro S, King TP, Shirazi-Beechey SP (2003) Glucose sensing in the intestinal epithelium. Eur J Biochem 270:3377–3388
Dyer J, Vayro S, Shirazi-Beechey SP (2003) Mechanism of glucose sensing in the small intestine. Biochem Soc Trans 31:1140–1142. https://doi.org/10.1042/
Ferraris RP (2001) Dietary and developmental regulation of intestinal sugar transport. Biochem J 360:265–276
Ferraris RP, Diamond J (1997) Regulation of intestinal sugar transport. Physiol Rev 77:257–302
Ferraris RP, Diamond JM (1989) Specific regulation of intestinal nutrient transporters by their dietary substrates. Annu Rev Physiol 51:125–141
Furness JB, Kunze WA, Clerc N (1999) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Phys 277:G922–G928
Ganapathy V, Gupta N, Martindale R (2006) Protein digestion and absorption. In: Johnson LR (ed) Physiology of the gastrointestinal tract, Fourth edn. Academic Press, pp 1667–1692
Grundy D, Scratcherd T (1989) Sensory afferents from the gastrointestinal tract. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation, vol I pt I. American Physiological Society, Bethesda, MD, pp 593–620
Guimbaud R, Moreau JA, Bouisson M, Durand S, Escourrou J, Vaysse N, Frexinos J (1997) Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas 14:76–82
Hammond DL, Ruda MA (1991) Developmental alterations in nociceptive threshold, immunoreactive calcitonin gene-related peptide and substance P, and fluoride-resistant acid phosphatase in neonatally capsaicin-treated rats. J Comp Neurol 312:436–450
Hirsh AJ, Tsang R, Kammila S, Cheeseman CI (1996) Effect of cholecystokinin and related peptides on jejunal transepithelial hexose transport in the Sprague-Dawley rat. Am J Phys 271:G755–G761
Holzer HH, Turkelson CM, Solomon TE, Raybould HE (1994) Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Phys 267:G625–G629
Jodal M, Holmgren S, Lundgren O, Sjoqvist A (1993) Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology 105:1286–1293
Kellett GL, Brot-Laroche E, Mace OJ, Leturque A (2008) Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28:35–54. https://doi.org/10.1146/annurev.nutr.28.061807.155518
Kimura Y, Turner JR, Braasch DA, Buddington RK (2005) Lumenal adenosine and AMP rapidly increase glucose transport by intact small intestine. Am J Physiol Gastrointest Liver Physiol 289:G1007–G1014. https://doi.org/10.1152/ajpgi.00085.2005
McCafferty DM, Wallace JL, Sharkey KA (1997) Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Phys 272:G272–G280
Mei N (1978) Vagal glucoreceptors in the small intestine of the cat. J Physiol 282:485–506
Migrenne S, Marsollier N, Cruciani-Guglielmacci C, Magnan C (2006) Importance of the gut-brain axis in the control of glucose homeostasis. Curr Opin Pharmacol 6:592–597. https://doi.org/10.1016/j.coph.2006.08.004
Mourad FH, Barada KA, Khoury C, Hamdi T, Saade NE, Nassar CF (2009) Amino acids in the rat intestinal lumen regulate their own absorption from a distant intestinal site. Am J Physiol Gastrointest Liver Physiol 297:G292–G298. https://doi.org/10.1152/ajpgi.00100.2009
Nassar CF, Abdallah LE, Barada KA, Atweh SF, Saade NE (1995) Effects of intravenous vasoactive intestinal peptide injection on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept 55:261–267
Nellgard P, Jonsson A, Bojo L, Tarnow P, Cassuto J (1996) Small-bowel obstruction and the effects of lidocaine, atropine and hexamethonium on inflammation and fluid losses. Acta Anaesthesiol Scand 40:287–292
Philpott DJ, Butzner JD, Meddings JB (1992) Regulation of intestinal glucose transport. Can J Physiol Pharmacol 70:1201–1207
Raybould HE (1999) Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Phys 277:G751–G755
Raybould HE, Holzer H (1992) Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett 141:236–238
Roder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 9:e89977. https://doi.org/10.1371/journal.pone.0089977
Rolston DD, Borodo MM, Kelly MJ, Dawson AM, Farthing MJ (1987) Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. J Pediatr Gastroenterol Nutr 6:624–630
Safieh-Garabedian B, Poole S, Haddad JJ, Massaad CA, Jabbur SJ, Saade NE (2002) The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology 42:864–872
Sayegh AI, Covasa M, Ritter RC (2004) Intestinal infusions of oleate and glucose activate distinct enteric neurons in the rat. Auton Neurosci 115:54–63
Schedl HP (1966) Use of polyethylene glycol and phenol red as unabsorbed indicators for intestinal absorption studies in man. Gut 7:159–163
Sharp PA, Debnam ES, Srai SK (1996) Rapid enhancement of brush border glucose uptake after exposure of rat jejunal mucosa to glucose. Gut 39:545–550
Shirazi-Beechey SP, Dyer J, Allison G, Wood IS (1996) Nutrient regulation of intestinal sugar-transporter expression. Biochem Soc Trans 24:389–392
Shirazi-Beechey SP, Hirayama BA, Wang Y, Scott D, Smith MW, Wright EM (1991) Ontogenic development of lamb intestinal sodium-glucose co-transporter is regulated by diet. J Physiol 437:699–708
Solberg DH, Diamond JM (1987) Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Phys 252:G574–G584
Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A (2010) Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 251:865–871. https://doi.org/10.1097/SLA.0b013e3181d96e1f
Stumpel F, Burcelin R, Jungermann K, Thorens B (2001) Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A 98:11330–11335. https://doi.org/10.1073/pnas.211357698
Sun Y, Fihn BM, Sjovall H, Jodal M (2004) Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut 53:362–367
Weiss SL, Lee EA, Diamond J (1998) Evolutionary matches of enzyme and transporter capacities to dietary substrate loads in the intestinal brush border. Proc Natl Acad Sci U S A 95:2117–2121
Wright EM, Hirsch JR, Loo DD, Zampighi GA (1997) Regulation of Na+/glucose cotransporters. J Exp Biol 200:287–293
Zimmerman TW, Binder HJ (1983) Effect of tetrodotoxin on cholinergic agonist-mediated colonic electrolyte transport. Am J Phys 244:G386–G391
Zittel TT, Rothenhofer I, Meyer JH, Raybould HE (1994) Small intestinal capsaicin-sensitive afferents mediate feedback inhibition of gastric emptying in rats. Am J Phys 267:G1142–G1145
Acknowledgments
The research was supported by a grant from the University Research Board (URB) and the Medical Practice Plan (MPP) of the American University of Beirut, Lebanon.
Author information
Authors and Affiliations
Contributions
FD and KB contributed in study design, analyzing the data, and writing the paper. NS contributed in performing the research, analyzing the data, and writing the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the American University of Beirut and were approved by its Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Mourad, F.H., Barada, K.A. & Saade, N.E. Inhibitory effect of luminal saccharides on glucose absorption from an adjacent jejunal site in rats: a newly described intestinal neural reflex. Pflugers Arch - Eur J Physiol 471, 595–603 (2019). https://doi.org/10.1007/s00424-018-2230-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2230-0