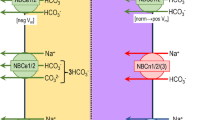
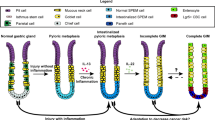
Abstract
One of the cardinal symptoms of intestinal inflammation is diarrhea. Acute intestinal inflammation is associated with inhibition of ion absorption and increased secretion, along with fluid leakage due to epithelial injury and changes in permeability. However, in the chronic situation, a downregulation of both absorptive and secretory transport has been reported. We investigated how experimental colitis reduces cAMP levels in intestinal epithelial cells through modulation of adenylyl cyclases (AC). Primary colonic epithelial cells obtained from rats with trinitrobenzenesulfonic acid colitis and non-colitic controls were analyzed for AC expression by RT-qPCR and Western blot, following a preliminary microarray analysis. AC6 and AC5 were found to be expressed in colonocytes, and downregulated by inflammation, with the former exhibiting considerably higher mRNA levels in both cases. To test the hypothesis that inflammatory cytokines may account for this effect, Caco 2 cells were treated with IL-1β, TNF-α, or IFN-γ. All three cytokines inhibited forskolin evoked short-circuit currents in Ussing chambers and lowered intracellular cAMP, but failed to alter AC6 mRNA levels. AC5/AC6 expression was however inhibited in mouse jejunal organoids treated with IFN-γ and TNF-α, but not IL-1β. Gene knockdown of AC6 resulted in a significant decrease of ion secretion in T84 cells. We conclude that the disturbances in ion secretion observed in rat TNBS colitis are associated with low intracellular levels of cAMP in the epithelium, which may be explained in part by the downregulation of AC5/AC6 expression by proinflammatory cytokines.







Similar content being viewed by others
References
Asfaha S, Bell CJ, Wallace JL, MacNaughton WK (1999) Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Phys 276:G703–G710
Barrett KE, Keely SJ (2000) Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62:535–572
Bell CJ, Gall DG, Wallace JL (1995) Disruption of colonic electrolyte transport in experimental colitis. Am J Phys 268:G622–G630
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M (2001) Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 29:365–371
Crowe SE, Luthra GK, Perdue MH (1997) Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut 41:785–792
Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945
Fan YY, Davidson LA, Chapkin RS (2016) Murine colonic organoid culture system and downstream assay applications. Methods Mol Humana Press Biol. https://doi.org/10.1007/7651_2016_8
Field M (2003) Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111:931–943
Goldhill JM, Burakoff R, Donovan V, Rose K, Percy WH (1993) Defective modulation of colonic secretomotor neurons in a rabbit model of colitis. Am J Phys 264:G671–G677
Greig E, Sandle GI (2000) Diarrhea in ulcerative colitis. The role of altered colonic sodium transport. Ann N Y Acad Sci 915:327–332
Hanoune J, Defer N (2001) Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174
Hawker PC, McKay JS, Turnberg LA (1980) Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology 79:508–511
Hubel KA, Renquist KS (1990) Ion transport in normal and inflamed human jejunum in vitro. Changes with electric field stimulation and theophylline. Dig Dis Sci 35:815–820
Jenkins HR, Milla PJ (1993) The effect of colitis on large-intestinal electrolyte transport in early childhood. J Pediatr Gastroenterol Nutr 16:402–405
Kachur JF, Keshavarzian A, Sundaresan R, Doria M, Walsh R, de las Alas MM, Gaginella TS (1995) Colitis reduces short-circuit current response to inflammatory mediators in rat colonic mucosa. Inflammation 19:245–259
Martinez-Augustin O, Merlos M, Zarzuelo A, Suarez MD, Sanchez de Medina F (2008) Disturbances in metabolic, transport and structural genes in experimental colonic inflammation in the rat: a longitudinal genomic analysis. BMC Genomics 9:490
Martinez-Augustin O, Romero-Calvo I, Suarez MD, Zarzuelo A, Sanchez de Medina F (2009) Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis 15:114–127
Martinez-Moya P, Ortega-Gonzalez M, Gonzalez R, Anzola A, Ocon B, Hernandez-Chirlaque C, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F (2012) Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol Res 66:144–153
O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW (2002) Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol 185:137–144
Pérez-Navarro R, Ballester I, Zarzuelo A, Sánchez de Medina F (2005) Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci 76:1489–1501
Perez-Navarro R, Martinez-Augustin O, Ballester I, Zarzuelo A, Sanchez de Medina F (2005) Experimental inflammation of the rat distal colon inhibits ion secretion in the proximal colon by affecting the enteric nervous system. Naunyn Schmiedeberg's Arch Pharmacol 371:114–121
Romero-Calvo I, Mascaraque C, Zarzuelo A, Suarez MD, Martinez-Augustin O, de Medina FS (2011) Intestinal inflammation and the enterocyte transportome. Biochem Soc Trans 39:1096–1101
Sanchez de Medina F, Perez R, Martinez-Augustin O, Gonzalez R, Lorente MD, Galvez J, Zarzuelo A (2002) Disturbances of colonic ion secretion in inflammation: role of the enteric nervous system and cAMP. Pflugers Arch 444:378–388
Sandle GI, Rajendran VM (2012) Cyclic AMP-induced K+ secretion occurs independently of Cl- secretion in rat distal colon. Am J Physiol Cell Physiol 303:C328–C333
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Skinn AC, MacNaughton WK (2005) Nitric oxide inhibits cAMP-dependent CFTR trafficking in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 289:G739–G744
Sunahara RK, Taussig R (2002) Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2:168–184
Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK (2008) Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117:61–69
Uribe JM, McCole DF, Barrett KE (2002) Interferon-gamma activates EGF receptor and increases TGF-alpha in T84 cells: implications for chloride secretion. Am J Physiol Gastrointest Liver Physiol 283:G923–G931
Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL (2003) Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol 285:G850–G860
Yoo BK, Yanda MK, No YR, Yun CC (2012) Human intestinal epithelial cell line SK-CO15 is a new model system to study Na(+)/H(+) exchanger 3. Am J Physiol Gastrointest Liver Physiol 303:G180–G188
Acknowledgements
The authors want to express their gratitude to Samuel Cantarero and the Centro de Instrumentación Científica of the University of Granada. This work was supported by the Ministry of Economy and Competitivity, partly with Fondo Europeo de Desarrollo Regional FEDER funds [grant numbers SAF2008-01432, AGL2008-04332, SAF2011-22922, SAF2011-22812, BFU2014-57736-P, AGL2014-58883-R]; and by Junta de Andalucía [grants number CTS164, CTS235]. IRC, BO, RGB and CHC were funded by Ministry of Education. CIBERehd is funded by the Instituto de Salud Carlos III. We also appreciate the collaboration of the Plataforma Andaluza de Bioinformática.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOC 52 kb)
Supplementary Table 2
(DOC 59 kb)
Supplementary Table 3
(DOC 120 kb)
Supplementary Table 4
(DOCX 12 kb)
Supplementary Fig. 1
Chromatogram example of a standard (a) and sample (b) for cAMP. (PNG 297 kb)
Supplementary Fig. 2
Schematic representation of the effect of TNBS on gene expression in rat colonocytes. (PNG 140 kb)
Supplementary Fig. 3
Effects of IL-1β, TNF-α or IFN-γ on IL-8 and MCP-1 production in Caco 2 cells after 25 h incubation. n = 6. +p < 0.05 vs. control. (PNG 36 kb)
Supplementary Fig. 4
Expression of ATPase α and β subunits, Cftr, Nkcc1, Nhe3 and Dra (a-f, respectively) in jejunum organoids from WT mice studied by RT-qPCR. n = 8–10. +p < 0.05 vs. control. (PNG 188 kb)
Rights and permissions
About this article
Cite this article
Romero-Calvo, I., Ocón, B., Gámez-Belmonte, R. et al. Adenylyl cyclase 6 is involved in the hyposecretory status of experimental colitis. Pflugers Arch - Eur J Physiol 470, 1705–1717 (2018). https://doi.org/10.1007/s00424-018-2187-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2187-z