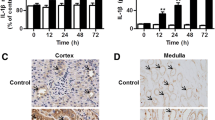
Abstract
Endotoxemia-related acute kidney injury (AKI) is associated with increased formation of prostaglandins, which may serve as a compensatory mechanism to maintain renal function. We hypothesized that an increase of renal EP2 or EP4 receptors and/or a downregulation of renal EP1 and EP3 receptors enhances PGE2-induced renal vasodilatation. Injection of lipopolysaccharide (LPS; 3 mg/kg i.p.) increased microsomal prostaglandin E synthase (mPGES)-1 and prostacyclin synthase expression, whereas mPGES-2 expression was unaltered. Further, LPS increased the mRNA abundance for the prostaglandin EP4 receptor, whereas the expressions of the EP1 and EP3 receptors were decreased. In isolated-perfused kidneys from control mice, PGE2 exerted a dual effect on renal vascular tone, inducing vasodilatation at lower concentrations and vasoconstriction at higher concentrations. In kidneys from endotoxemic mice, the vasodilatory component was more pronounced, whereas the vasoconstriction at higher PGE2 concentrations was absent. Similarly, prostacyclin (PGI2)-induced vasodilatation was more pronounced in endotoxemic kidneys. The enhanced vasodilatory effect was paralleled by an increase in renal vascular EP4 and prostacyclin IP receptor mRNA expression. Further, stimulation of renin secretion rate by PGE2 and PGI2 was enhanced in endotoxemic kidneys. Pretreatment with the cyclooxygenase (COX)-2 inhibitor SC-236 (10 mg/kg) did not alter the basal GFR, but augmented the LPS-induced decline in GFR, and attenuated the LPS-induced increase in plasma renin concentration in vivo. Our data suggest that an activation of the COX-2/mPGES-1 synthetic pathway is responsible for the increased renal formation of PGE2 in response to LPS and that the vasodilatory effect of PGE2 and PGI2 is enhanced during endotoxemia.








Similar content being viewed by others
References
Hao CM, Breyer MD (2008) Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70:357–377. https://doi.org/10.1146/annurev.physiol.70.113006.100614
Brater DC (2002) Renal effects of cyclooxygyenase-2-selective inhibitors. J Pain Symptom Manag 23:S15–S20 discussion S21–3
Clive DM, Stoff JS (1984) Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med 310:563–572. https://doi.org/10.1056/NEJM198403013100905
Ciabattoni G, Cinotti GA, Pierucci A, Simonetti BM, Manzi M, Pugliese F, Barsotti P, Pecci G, Taggi F, Patrono C (1984) Effects of sulindac and ibuprofen in patients with chronic glomerular disease. Evidence for the dependence of renal function on prostacyclin. N Engl J Med 310:279–283. https://doi.org/10.1056/NEJM198402023100502
Tang L, Loutzenhiser K, Loutzenhiser R (2000) Biphasic actions of prostaglandin E(2) on the renal afferent arteriole : role of EP(3) and EP(4) receptors. Circ Res 86:663–670
Eskildsen MP, Hansen PB, Stubbe J, Toft A, Walter S, Marcussen N, Rasmussen LM, Vanhoutte PM, Jensen BL (2014) Prostaglandin I2 and prostaglandin E2 modulate human intrarenal artery contractility through prostaglandin E2-EP4, prostacyclin-IP, and thromboxane A2-TP receptors. Hypertension 64:551–556. https://doi.org/10.1161/HYPERTENSIONAHA.113.03051
Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A (2004) Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287:F427–F433. https://doi.org/10.1152/ajprenal.00072.2004
Morath R, Klein T, Seyberth HW, Nusing RM (1999) Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol 10:1851–1860
Komhoff M, Lesener B, Nakao K, Seyberth HW, Nusing RM (1998) Localization of the prostacyclin receptor in human kidney. Kidney Int 54:1899–1908. https://doi.org/10.1046/j.1523-1755.1998.00213.x
Perner A, Cecconi M, Cronhjort M, Darmon M, Jakob SM, Pettila V, van der Horst ICC (2018) Expert statement for the management of hypovolemia in sepsis. Intensive Care Med 44:791–798. https://doi.org/10.1007/s00134-018-5177-x
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. https://doi.org/10.1001/jama.2016.0287
Opal SM (2010) Endotoxins and other sepsis triggers. Contrib Nephrol 167:14–24. https://doi.org/10.1159/000315915
Levy B, Fritz C, Tahon E, Jacquot A, Auchet T, Kimmoun A (2018) Vasoplegia treatments: the past, the present, and the future. Crit Care 22:52. https://doi.org/10.1186/s13054-018-1967-3
Schmidt C, Hocherl K, Kurt B, Bucher M (2008) Role of nuclear factor-kappaB-dependent induction of cytokines in the regulation of vasopressin V1A-receptors during cecal ligation and puncture-induced circulatory failure. Crit Care Med 36:2363–2372. https://doi.org/10.1097/CCM.0b013e318180b51d
Schmidt C, Hocherl K, Kurt B, Moritz S, Kurtz A, Bucher M (2010) Blockade of multiple but not single cytokines abrogates downregulation of angiotensin II type-I receptors and anticipates septic shock. Cytokine 49:30–38. https://doi.org/10.1016/j.cyto.2009.10.006
Schmidt C, Kurt B, Hocherl K, Bucher M (2009) Inhibition of NF-kappaB activity prevents downregulation of alpha1-adrenergic receptors and circulatory failure during CLP-induced sepsis. Shock 32:239–246. https://doi.org/10.1097/SHK.0b013e3181994752
Hocherl K, Dreher F, Kurtz A, Bucher M (2002) Cyclooxygenase-2 inhibition attenuates lipopolysaccharide-induced cardiovascular failure. Hypertension 40:947–953
Hocherl K, Schmidt C, Kurt B, Bucher M (2008) Activation of the PGI(2)/IP system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension 52:330–335. https://doi.org/10.1161/HYPERTENSIONAHA.108.112029
Szabo C, Southan GJ, Thiemermann C (1994) Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci U S A 91:12472–12476
Schrier RW, Wang W (2004) Acute renal failure and sepsis. N Engl J Med 351:159–169. https://doi.org/10.1056/NEJMra032401
Lipcsey M, Bellomo R (2011) Septic acute kidney injury: hemodynamic syndrome, inflammatory disorder, or both? Crit Care 15:1008. https://doi.org/10.1186/cc10525
Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A (2017) Acute kidney injury in sepsis. Intensive Care Med 43:816–828. https://doi.org/10.1007/s00134-017-4755-7
van Lambalgen AA, Bouriquet N, Casellas D (1996) Effects of endotoxin on tone and pressure-responsiveness of preglomerular juxtamedullary vessels. Pflugers Arch 432:574–577
Lugon JR, Boim MA, Ramos OL, Ajzen H, Schor N (1989) Renal function and glomerular hemodynamics in male endotoxemic rats. Kidney Int 36:570–575
Van Beusecum JP, Zhang S, Cook AK, Inscho EW (2017) Acute toll-like receptor 4 activation impairs rat renal microvascular autoregulatory behaviour. Acta Physiol (Oxf) 221:204–220. https://doi.org/10.1111/apha.12899
El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC (2006) Sepsis induces changes in the expression and distribution of toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290:F1034–F1043. https://doi.org/10.1152/ajprenal.00414.2005
Bucher M, Kees F, Taeger K, Kurtz A (2003) Cytokines down-regulate alpha1-adrenergic receptor expression during endotoxemia. Crit Care Med 31:566–571. https://doi.org/10.1097/01.CCM.0000048621.36569.69
Yamaguchi N, Jesmin S, Zaedi S, Shimojo N, Maeda S, Gando S, Koyama A, Miyauchi T (2006) Time-dependent expression of renal vaso-regulatory molecules in LPS-induced endotoxemia in rat. Peptides 27:2258–2270. https://doi.org/10.1016/j.peptides.2006.03.025
Boffa JJ, Arendshorst WJ (2005) Maintenance of renal vascular reactivity contributes to acute renal failure during endotoxemic shock. J Am Soc Nephrol 16:117–124. https://doi.org/10.1681/ASN.2004060441
Hocherl K, Schmidt C, Bucher M (2009) COX-2 inhibition attenuates endotoxin-induced downregulation of organic anion transporters in the rat renal cortex. Kidney Int 75:373–380. https://doi.org/10.1038/ki.2008.557
Boulet L, Ouellet M, Bateman KP, Ethier D, Percival MD, Riendeau D, Mancini JA, Methot N (2004) Deletion of microsomal prostaglandin E2 (PGE2) synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J Biol Chem 279:23229–23237. https://doi.org/10.1074/jbc.M400443200
Feng L, Sun W, Xia Y, Tang WW, Chanmugam P, Soyoola E, Wilson CB, Hwang D (1993) Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch Biochem Biophys 307:361–368
Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S (1991) Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 349:617–620. https://doi.org/10.1038/349617a0
Boffa JJ, Just A, Coffman TM, Arendshorst WJ (2004) Thromboxane receptor mediates renal vasoconstriction and contributes to acute renal failure in endotoxemic mice. J Am Soc Nephrol 15:2358–2365. https://doi.org/10.1097/01.ASN.0000136300.72480.86
Mederle K, Meurer M, Castrop H, Hocherl K (2015) Inhibition of COX-1 attenuates the formation of thromboxane A2 and ameliorates the acute decrease in glomerular filtration rate in endotoxemic mice. Am J Physiol Renal Physiol 309:F332–F340. https://doi.org/10.1152/ajprenal.00567.2014
Johannes T, Ince C, Klingel K, Unertl KE, Mik EG (2009) Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit Care Med 37:1423–1432. https://doi.org/10.1097/CCM.0b013e31819b5f4e
Wang W, Zolty E, Falk S, Summer S, Stearman R, Geraci M, Schrier R (2007) Prostacyclin in endotoxemia-induced acute kidney injury: cyclooxygenase inhibition and renal prostacyclin synthase transgenic mice. Am J Physiol Renal Physiol 293:F1131–F1136. https://doi.org/10.1152/ajprenal.00212.2007
Henrich WL, Hamasaki Y, Said SI, Campbell WB, Cronin RE (1982) Dissociation of systemic and renal effects in endotoxemia. Prostaglandin inhibition uncovers an important role of renal nerves. J Clin Invest 69:691–699
Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP (1998) Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Phys 274:F481–F489
Hocherl K, Schmidt C, Kurt B, Bucher M (2010) Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol 298:F196–F204. https://doi.org/10.1152/ajprenal.90607.2008
Schmidt C, Hocherl K, Schweda F, Bucher M (2007) Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Crit Care Med 35:2110–2119
Zarjou A, Agarwal A (2011) Sepsis and acute kidney injury. J Am Soc Nephrol 22:999–1006. https://doi.org/10.1681/ASN.2010050484
Kikeri D, Pennell JP, Hwang KH, Jacob AI, Richman AV, Bourgoignie JJ (1986) Endotoxemic acute renal failure in awake rats. Am J Phys 250:F1098–F1106. https://doi.org/10.1152/ajprenal.1986.250.6.F1098
Badr KF, Kelley VE, Rennke HG, Brenner BM (1986) Roles for thromboxane A2 and leukotrienes in endotoxin-induced acute renal failure. Kidney Int 30:474–480
Li T, Liu Y, Zhao J, Miao S, Xu Y, Liu K, Liu M, Wang G, Xiao X (2017) Aggravation of acute kidney injury by mPGES-2 down regulation is associated with autophagy inhibition and enhanced apoptosis. Sci Rep 7:10247. https://doi.org/10.1038/s41598-017-10271-8
Inscho EW, Carmines PK, Navar LG (1990) Prostaglandin influences on afferent arteriolar responses to vasoconstrictor agonists. Am J Phys 259:F157–F163. https://doi.org/10.1152/ajprenal.1990.259.1.F157
Edwards RM (1985) Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Phys 248:F779–F784. https://doi.org/10.1152/ajprenal.1985.248.6.F779
Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM (2000) The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta 1483:285–293
Khan RZ, Badr KF (1999) Endotoxin and renal function: perspectives to the understanding of septic acute renal failure and toxic shock. Nephrol Dial Transplant 14:814–818
Leach M, Hamilton LC, Olbrich A, Wray GM, Thiemermann C (1998) Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br J Pharmacol 124:586–592. https://doi.org/10.1038/sj.bjp.0701869
Tunctan B, Korkmaz B, Cuez T, Kemal Buharalioglu C, Sahan-Firat S, Falck J, Malik KU (2010) Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase-2 inhibitor, NS-398, on endotoxin-induced hypotension in rats. Basic Clin Pharmacol Toxicol 107:877–882. https://doi.org/10.1111/j.1742-7843.2010.00589.x
Azab AN, Kobal S, Rubin M, Kaplanski J (2005) Effects of nimesulide, a selective cyclooxygenase-2 inhibitor, on cardiovascular alterations in endotoxemia. Cardiology 103:92–100. https://doi.org/10.1159/000082470
Reddy RC, Chen GH, Tateda K, Tsai WC, Phare SM, Mancuso P, Peters-Golden M, Standiford TJ (2001) Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am J Physiol Lung Cell Mol Physiol 281:L537–L543. https://doi.org/10.1152/ajplung.2001.281.3.L537
Staehr M, Madsen K, Vanhoutte PM, Hansen PB, Jensen BL (2011) Disruption of COX-2 and eNOS does not confer protection from cardiovascular failure in lipopolysaccharide-treated conscious mice and isolated vascular rings. Am J Physiol Regul Integr Comp Physiol 301:R412–R420. https://doi.org/10.1152/ajpregu.00823.2010
Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA (2009) Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 20:1217–1221. https://doi.org/10.1681/ASN.2008060617
Gerber JG, Keller RT, Nies AS (1979) Prostaglandins and renin release: the effect of PGI2, PGE2, and 13,14-dihydro PGE2 on the baroreceptor mechanism of renin release in the dog. Circ Res 44:796–799
Franco-Saenz R, Suzuki S, Tan SY, Mulrow PJ (1980) Prostaglandin stimulation of renin release: independence of beta-adrenergic receptor activity and possible mechanism of action. Endocrinology 106:1400–1404. https://doi.org/10.1210/endo-106-5-1400
Ito S, Carretero OA, Abe K, Beierwaltes WH, Yoshinaga K (1989) Effect of prostanoids on renin release from rabbit afferent arterioles with and without macula densa. Kidney Int 35:1138–1144
Henrich WL, Campbell WB (1984) Relationship between PG and beta-adrenergic pathways to renin release in rat renal cortical slices. Am J Phys 247:E343–E348. https://doi.org/10.1152/ajpendo.1984.247.3.E343
Hackenthal E, Schwertschlag U, Seyberth HW (1980) Prostaglandins and renin release studies in the isolated perfused rat kidney. Prog Biochem Pharmacol 17:98–107
Jensen BL, Schmid C, Kurtz A (1996) Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Phys 271:F659–F669. https://doi.org/10.1152/ajprenal.1996.271.3.F659
Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nusing RM, Skott O, Jensen BL (2005) Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol 289:F989–F997. https://doi.org/10.1152/ajprenal.00201.2005
Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M (2007) Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18:1072–1083. https://doi.org/10.1681/ASN.2006050454
Doerschug KC, Delsing AS, Schmidt GA, Ashare A (2010) Renin-angiotensin system activation correlates with microvascular dysfunction in a prospective cohort study of clinical sepsis. Crit Care 14:R24. https://doi.org/10.1186/cc8887
Hilgenfeldt U, Kienapfel G, Kellermann W, Schott R, Schmidt M (1987) Renin-angiotensin system in sepsis. Clin Exp Hypertens A 9:1493–1504
Ohtani R, Ohashi Y, Muranaga K, Itoh N, Okamoto H (1989) Changes in activity of the renin-angiotensin system of the rat by induction of acute inflammation. Life Sci 44:237–241
Todorov V, Muller M, Schweda F, Kurtz A (2002) Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol 283:R1046–R1051. https://doi.org/10.1152/ajpregu.00142.2002
Antonipillai I, Wang Y, Horton R (1990) Tumor necrosis factor and interleukin-1 may regulate renin secretion. Endocrinology 126:273–278. https://doi.org/10.1210/endo-126-1-273
Burnier M, Waeber B, Aubert JF, Nussberger J, Brunner HR (1988) Effects of nonhypotensive endotoxemia in conscious rats: role of prostaglandins. Am J Phys 254:H509–H516. https://doi.org/10.1152/ajpheart.1988.254.3.H509
Nitescu N, DiBona GF, Grimberg E, Guron G (2010) Angiotensin II type 1 receptor antagonism attenuates abnormalities in dynamic renal blood flow autoregulation in rats with endotoxin-induced acute kidney injury. Kidney Blood Press Res 33:200–208. https://doi.org/10.1159/000316705
Nitescu N, Grimberg E, Guron G (2008) Low-dose candesartan improves renal blood flow and kidney oxygen tension in rats with endotoxin-induced acute kidney dysfunction. Shock 30:166–172. https://doi.org/10.1097/shk.0b013e31815dd780
Heyman SN, Darmon D, Goldfarb M, Bitz H, Shina A, Rosen S, Brezis M (2000) Endotoxin-induced renal failure. I A role for altered renal microcirculation. Exp Nephrol 8:266–274. https://doi.org/10.1159/000020678
Risoe PK, Wang Y, Stuestol JF, Aasen AO, Wang JE, Dahle MK (2007) Lipopolysaccharide attenuates mRNA levels of several adenylyl cyclase isoforms in vivo. Biochim Biophys Acta 1772:32–39. https://doi.org/10.1016/j.bbadis.2006.08.007
Choi WI, Kwon KY, Seo JW, Beagle J, Quinn DA, Hales CA (2009) The role of phosphodiesterase 3 in endotoxin-induced acute kidney injury. BMC Infect Dis 9:80. https://doi.org/10.1186/1471-2334-9-80
Acknowledgements
The technical assistance provided by Ramona Mogge is gratefully acknowledged.
Funding
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, SFB699/B5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local animal protection committee.
Rights and permissions
About this article
Cite this article
Meurer, M., Ebert, K., Schweda, F. et al. The renal vasodilatory effect of prostaglandins is ameliorated in isolated-perfused kidneys of endotoxemic mice. Pflugers Arch - Eur J Physiol 470, 1691–1703 (2018). https://doi.org/10.1007/s00424-018-2183-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2183-3