Abstract
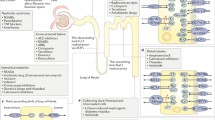
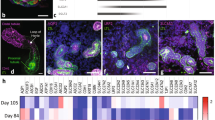
Given currently poor toxicity translational predictions for drug candidates, improved mechanistic understanding underlying nephrotoxicity and drug renal clearance is needed to improve drug development and safety screening. Therefore, better relevant and well-characterized in vitro screening models are required to reliably predict human nephrotoxicity. Because kidney proximal tubules are central to active drug uptake and secretion processes and therefore to nephrotoxicity, this study acquired regio-specific expression data from recently reported primary proximal tubule three-dimensional (3D) hyaluronic acid gel culture and non-gel embedded cultured murine proximal tubule suspensions used in nephrotoxicity assays. Quantitative assessment of the mRNA expression of 21 known kidney tubule markers and important proximal tubule transporters with known roles in drug transport was obtained. Asserting superior gene expression levels over current commonly used two-dimensional (2D) kidney cell culture lines was the study objective. Hence, we compare previously published gel-based 3D proximal tubule fragment culture and their non-gel suspensions for up to 1 week. We demonstrate that 3D tubule culture exhibits superior gene expression levels and profiles compared to published commonly used 2D kidney cell lines (Caki-1 and HK-2) in plastic plate monocultures. Additionally, nearly all tested genes retain mRNA expression after 7 days in both proximal tubule cultures, a limitation of 2D cell culture lines. Importantly, gel presence is shown not to interfere with the gene expression assay. Western blots confirm protein expression of OAT1 and 3 and OCT2. Functional transport assays confirm their respective transporter functions in vitro. Overall, results validate retention of essential toxicity-relevant transporters in this published 3D proximal tubule model over conventional 2D kidney cell cultures, producing opportunities for more reliable, sensitive, and comprehensive drug toxicity studies relevant to drug development and nephrotoxicity goals.







Similar content being viewed by others
References
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496
Anzai N, Endou H (2007) Renal drug transporters and nephrotoxicity. AATEX 14:447–452
Aschauer L, Wilmes A, Limonciel A, Leonard MO, Pfaller W, Jennings P (2014) Application of a human renal proximal tubule cell line, RPTEC/TERT1, for chemical safety assessment. Toxicol Lett 229:S242–S243. https://doi.org/10.1016/j.toxlet.2014.06.809
Aschauer L, Carta G, Vogelsang N, Schlatter E, Jennings P (2015) Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol in Vitro 30:95–105
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69-70:1–18. https://doi.org/10.1016/j.addr.2014.02.008
Astashkina A, Mann B, Grainger DW (2012) A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther 134:82–106. https://doi.org/10.1016/j.pharmthera.2012.01.001
Astashkina AI, Mann BK, Prestwich GD, Grainger DW (2012) A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials 33:4700–4711. https://doi.org/10.1016/j.biomaterials.2012.02.063
Astashkina AI, Mann BK, Prestwich GD, Grainger DW (2012) Comparing predictive drug nephrotoxicity biomarkers in kidney 3-D primary organoid culture and immortalized cell lines. Biomaterials 33:4712–4721. https://doi.org/10.1016/j.biomaterials.2012.03.001
Baharvand H, Hashemi SM, Ashtian SK, Farrokhi A (2006) Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol 50:645–652. https://doi.org/10.1387/ijdb.052072hb
Bhadriraju K, Chen CS (2002) Engineering cellular microenvironments to cell-based drug testing improve. Drug Discov Today 7:612–620. https://doi.org/10.1016/S1359-6446(02)02273-0
Birgersdotter A, Sandberg R, Ernberg I (2005) Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15:405–412. https://doi.org/10.1016/j.semcancer.2005.06.009
Brown CD, Sayer R, Windass AS, Haslam IS, De Broe ME, D'Haese PC, Verhulst A (2008) Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol Appl Pharmacol 233:428–438. https://doi.org/10.1016/j.taap.2008.09.018
Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota H-J, Haier J, Jaehde U, Zisowsky J, Schlatter E (2005) Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167:1477–1484
Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E (2010) Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol 176:1169–1180. https://doi.org/10.2353/ajpath.2010.090610
Crivellato E, Candussio L, Rosati AM, Decorti G, Klugmann FB, Mallardi F (1999) Kinetics of doxorubicin handling in the LLC-PK1 kidney epithelial cell line is mediated by both vesicle formation and P-glycoprotein drug transport. Histochem J 31:635–643. https://doi.org/10.1023/A:1003893218761
DesRochers TM, Suter L, Roth A, Kaplan DL (2013) Bioengineered 3D human kidney tissue, a platform for the determination of nephrotoxicity. PLoS One 8:e59219. https://doi.org/10.1371/journal.pone.0059219
Diekjürgen D (2017) Validating an ex vivo 3D kidney proximal tubule model for drug-induced nephrotoxicity screening. Doctoral dissertation, ProQuest Dissertations and Theses database
Diekjürgen D, Grainger DW (2018) An ex vivo 3D kidney proximal tubule model improves predictions of clinical drug-induced nephrotoxicity. Toxicol Appl Pharmacol, revised and in review
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12:207–218. https://doi.org/10.1089/adt.2014.573
Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, Sainz-Prestel V, Ortiz-Martin N, Parra-Rodriguez A, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A (2011) Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat 2011:11–11. https://doi.org/10.1155/2011/354908
Gallegos TF, Martovetsky G, Kouznetsova V, Bush KT, Nigam SK (2012) Organic anion and cation SLC22 “drug” transporter (Oat1, Oat3, and Oct1) regulation during development and maturation of the kidney proximal tubule. PLoS One 7:e40796
Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H (1997) Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 16:871–881
Greenburg G, Hay ED (1982) Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95:333–339
Gstraunthaler G, Pfaller W, Kotanko P (1985) Biochemical characterization of renal epithelial cell cultures (LLC-PK1 and MDCK). Am J Physiol Renal Physiol 248:F536–F544
Gunness P, Aleksa K, Kosuge K, Ito S, Koren G (2010) Comparison of the novel HK-2 human renal proximal tubular cell line with the standard LLC-PK1 cell line in studying drug-induced nephrotoxicity. Can J Physiol Pharmacol 88:448–455
Gurski LA, Petrelli NJ, Jia X, Farach-Carson MC (2010) 3D matrices for anti-cancer drug testing and development. Oncology Issues 25:20–25
Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J (2007) Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35:1333–1340. https://doi.org/10.1124/dmd.107.014902
Hosoyamada M, Sekine T, Kanai Y, Endou H (1999) Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol Renal Physiol 276:F122–F128
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21:745–754. https://doi.org/10.1016/j.tcb.2011.09.005
Huls M, Brown C, Windass A, Sayer R, Van Den Heuvel J, Heemskerk S, Russel F, Masereeuw R (2008) The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int 73:220–225
Inui K, Masuda S, Saito H (2000) Cellular and molecular aspects of drug transport in the kidney. Kidney Int 58:944–958. https://doi.org/10.1046/j.1523-1755.2000.00251.x
Inui K, Terada T, Masuda S, Saito H (2000) Physiological and pharmacological implications of peptide transporters, PEPT1 and PEPT2. Nephrol Dial Transplant 15:11–13. https://doi.org/10.1093/ndt/15.suppl_6.11
Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE (2013) Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb) 5:1119–1129. https://doi.org/10.1039/c3ib40049b
Jenkinson SE, Chung GW, van Loon E, Bakar NS, Dalzell AM, Brown CD (2012) The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflugers Arch 464:601–611. https://doi.org/10.1007/s00424-012-1163-2
Justice BA, Badr NA, Felder RA (2009) 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14:102–107. https://doi.org/10.1016/j.drudis.2008.11.006
Khaitan D, Chandna S, Arya MB, Dwarakanath BS (2006) Establishment and characterization of multicellular spheroids from a human glioma cell line; implications for tumor therapy. J Transl Med 4:12. https://doi.org/10.1186/1479-5876-4-12
Koepsell H, Endou H (2004) The SLC22 drug transporter family. Pflugers Arch 447:666–676. https://doi.org/10.1007/s00424-003-1089-9
Koyama H, Goodpasture C, Miller M, Teplitz R, Riggs AD (1978) Establishment and characterization of a cell line from the American opossum (Didelphys virginiana). In vitro 14:239–246
Lawrence ML, Chang CH, Davies JA (2015) Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys. Sci Rep 5:9092. https://doi.org/10.1038/srep09092
Lee J, Cuddihy MJ, Kotov NA (2008) Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14:61–86. https://doi.org/10.1089/teb.2007.0150
Li Y, Oo ZY, Chang SY, Huang P, Eng KG, Zeng JL, Kaestli AJ, Gopalan B, Kandasamy K, Tasnim F, Zink D (2013) An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol Res 2:352. https://doi.org/10.1039/c3tx50042j
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
McKim JM (2010) Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb Chem High Throughput Screen 13:188–206
Mehrens T, Lelleck S, Çetinkaya I, Knollmann M, Hohage H, Gorboulev V, Bokník P, Koepsell H, Schlatter E (2000) The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J Am Soc Nephrol 11:1216–1224
Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S (2012) Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release 164:192–204. https://doi.org/10.1016/j.jconrel.2012.04.045
Miller RL, Zhang P, Chen T, Rohrwasser A, Nelson RD (2006) Automated method for the isolation of collecting ducts. Am J Physiol Renal Physiol 291:F236–F245
Monien BH, Müller C, Bakhiya N, Donath C, Frank H, Seidel A, Glatt H (2009) Probenecid, an inhibitor of transmembrane organic anion transporters, alters tissue distribution of DNA adducts in 1-hydroxymethylpyrene-treated rats. Toxicology 262:80–85
Moss DM, Neary M, Owen A (2014) The role of drug transporters in the kidney: lessons from tenofovir. Front Pharmacol 5:248. https://doi.org/10.3389/fphar.2014.00248
Motoyoshi Y, Matsusaka T, Saito A, Pastan I, Willnow TE, Mizutani S, Ichikawa I (2008) Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int 74:1262–1269
Mutsaers HA, Wilmer MJ, van den Heuvel LP, Hoenderop JG, Masereeuw R (2011) Basolateral transport of the uraemic toxin p-cresyl sulfate: role for organic anion transporters? Nephrol Dial Transplant 26:4149. https://doi.org/10.1093/ndt/gfr562
Nelson CM, Bissell MJ (2005) Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 15:342–352. https://doi.org/10.1016/j.semcancer.2005.05.001
Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V (2015) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10:2039–2049. https://doi.org/10.2215/CJN.02440314
Nooter K, Westerman AM, Flens MJ, Zaman G, Scheper RJ, Van Wingerden K, Burger H, Oostrum R, Boersma T, Sonneveld P (1995) Expression of the multidrug resistance-associated protein (MRP) gene in human cancers. Clin Cancer Res 1:1301–1310
O'Brien LE, Zegers MM, Mostov KE (2002) Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3:531–537
Okuda M, Tsuda K, Masaki K, Hashimoto Y, Inui K (1999) Cisplatin-induced toxicity in LLC-PK1 kidney epithelial cells: role of basolateral membrane transport. Toxicol Lett 106:229–235. https://doi.org/10.1016/S0378-4274(99)00071-5
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Prestwich GD (2008) Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res 41:139–148. https://doi.org/10.1021/ar7000827
Prestwich GD (2011) Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release 155:193–199. https://doi.org/10.1016/j.jconrel.2011.04.007
Prestwich GD, Kuo JW (2008) Chemically-modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol 9:242–245
Price KJ, Tsykin A, Giles KM, Sladic RT, Epis MR, Ganss R, Goodall GJ, Leedman PJ (2012) Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem Biophys Res Commun 427:343–348. https://doi.org/10.1016/j.bbrc.2012.09.059
Provenzano PP, Eliceiri KW, Inman DR, Keely PJ (2010) Engineering three-dimensional collagen matrices to provide contact guidance during 3D cell migration. Curr Protoc Cell Biol:10.17. 11–10.17. 11
Race JE, Grassl SM, Williams WJ, Holtzman EJ (1999) Molecular cloning and characterization of two novel human renal organic anion transporters (hOAT1 and hOAT3). Biochem Bioph Res Co 255:508–514
Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR (2006) Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother 50:3297–3304
Rennick B (1981) Renal tubule transport of organic cations. Am J Physiol Renal Physiol 240:F83–F89
Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B (1994) HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45:48–57
Schor SL, Schor AM, Winn B, Rushton G (1982) The use of three-dimensional collagen gels for the study of tumour cell invasion in vitro: experimental parameters influencing cell migration into the gel matrix. Int J Cancer 29:57–62
Shitara Y, Sato H, Sugiyama Y (2005) Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol 45:689–723. https://doi.org/10.1146/annurev.pharmtox.44.101802.121444
Shitara Y, Horie T, Sugiyama Y (2006) Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci 27:425–446. https://doi.org/10.1016/j.ejps.2005.12.003
Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7:33. https://doi.org/10.1186/1471-2199-7-33
Snouber LC, Letourneur F, Chafey P, Broussard C, Monge M, Legallais C, Leclerc E (2012) Analysis of transcriptomic and proteomic profiles demonstrates improved Madin-Darby canine kidney cell function in a renal microfluidic biochip. Biotechnol Prog 28:474–484. https://doi.org/10.1002/btpr.743
Sweet DH, Eraly SA, Vaughn D, Bush K, Nigam S (2006) Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 69:837–845
Szot CS, Buchanan CF, Freeman JW, Rylander MN (2011) 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 32:7905–7912. https://doi.org/10.1016/j.biomaterials.2011.07.001
Terryn S, Jouret F, Vandenabeele F, Smolders I, Moreels M, Devuyst O, Steels P, Van Kerkhove E (2007) A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293:F476–F485. https://doi.org/10.1152/ajprenal.00363.2006
Thermo Fisher Scientific Inc. (2015) Thermo Fisher Scientific. www.thermofisher.com. 2015
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci 84:7735–7738
Tibbitt MW, Anseth KS (2009) Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103:655–663
Van der Hauwaert C, Savary G, Gnemmi V, Glowacki F, Pottier N, Bouillez A, Maboudou P, Zini L, Leroy X, Cauffiez C, Perrais M, Aubert S (2013) Isolation and characterization of a primary proximal tubular epithelial cell model from human kidney by CD10/CD13 double labeling. PLoS One 8:e66750. https://doi.org/10.1371/journal.pone.0066750
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034
Ward DT, McLarnon SJ, Riccardi D (2002) Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J Am Soc Nephrol 13:1481–1489
Weaver VM, Petersen OW, Wang F, Larabell C, Briand P, Damsky C, Bissell MJ (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137:231–245
Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R (2008) hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295:F1365–F1375
Wilde S, Schlatter E, Koepsell H, Edemir B, Reuter S, Pavenstadt H, Neugebauer U, Schroter R, Brast S, Ciarimboli G (2009) Calmodulin-associated post-translational regulation of rat organic cation transporter 2 in the kidney is gender dependent. Cell Mol Life Sci: CMLS 66:1729–1740. https://doi.org/10.1007/s00018-009-9145-z
Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, Mathieson PW, Monnens LA, van den Heuvel LP, Levtchenko EN (2010) Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res 339:449–457
Yang Z, Xiong H-R (2012) In vitro, tissue-based models as a replacement for animal models in testing of drugs at the preclinical stages. In: Ceccherini-Nelli L (ed) Biomedical tissue culture, chapter 5. InTech Publishing, pp 73–80. https://doi.org/10.5772/52300
Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K-I (2007) Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol 74:477–487
Yonezawa A, Inui K-I (2011) Organic cation transporter OCT/SLC22A and H+/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem Pharmacol 81:563–568
Yu LS, Shen Q, Zhou Q, Jiang HD, Bi HC, Huang M, Zhou H, Zeng S (2013) In vitro characterization of ABC transporters involved in the absorption and distribution of liensinine and its analogs. J Ethnopharmacol 150:485–491. https://doi.org/10.1016/j.jep.2013.08.061
Zietarska M, Maugard CM, Filali-Mouhim A, Alam-Fahmy M, Tonin PN, Provencher DM, Mes-Masson AM (2007) Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol Carcinog 46:872–885. https://doi.org/10.1002/mc.20315
Acknowledgments
This work was supported by the AstraZeneca UK Limited, London, England. We thank Dr. M. Wagoner at AstraZeneca Pharmaceuticals, USA, for valuable discussions. DWG is grateful for support from the George S. and Dolores Doré Eccles Foundation (USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Diekjürgen, D., Grainger, D.W. Drug transporter expression profiling in a three-dimensional kidney proximal tubule in vitro nephrotoxicity model. Pflugers Arch - Eur J Physiol 470, 1311–1323 (2018). https://doi.org/10.1007/s00424-018-2150-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2150-z