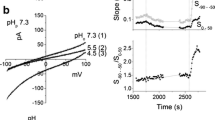
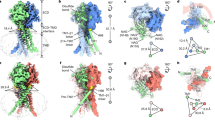
Abstract
A proton is a ubiquitous signaling ion. Many transmembrane H+ transport pathways either maintain pH homeostasis or generate acidic compartments. The osteoclast is a bone-resorbing cell, which degrades bone tissues by secreting protons and lysosomal enzymes into the resorption pit. The plasma membrane facing bone tissue (ruffled border), generated partly by fusion of lysosomes, may mimic H+ flux mechanisms regulating acidic vesicles. We identified three electrogenic H+-fluxes in osteoclast plasma membranes—a vacuolar H+-ATPase (V-ATPase), a voltage-gated proton channel (Hv channel) and an acid-inducible H+-leak—whose electrophysiological profiles and regulation mechanisms differed. V-ATPase and Hv channel, both may have intracellular reservoirs, but the recruitment/internalization is regulated independently. V-ATPase mediates active H+ efflux, acidifying the resorption pit, while acid-inducible H+ leak, activated at an extracellular pH < 5.5, diminishes pit acidification, possibly to protect bone from excess degradation. The two-way H+ flux mechanisms in opposite directions may have advantages in fine regulation of pit pH. Hv channel mediates passive H+ efflux. Although its working ranges are limited, the amount of H+ extrusion is 100 times larger than those of the V-ATPase and may support reactive oxygen species production during osteoclastogenesis. Extracellular Ca2+, H+ and inorganic phosphate, which accumulate in the resorption pit, will either stimulate or inhibit these H+ fluxes. Skeletal integration is disrupted by too much or too less of bone resorption. Diversities in plasma membrane H+ flux pathways, which may co-operate or compete, are essential to adjust osteoclast functions in variable conditions.





Similar content being viewed by others
Abbreviations
- V-ATPase:
-
Vacuolar H+-ATPase
- Hv channel:
-
Voltage-gated proton channel
- NHE:
-
Na+-H+ exchanger
- F-ATPase:
-
F-type H+-ATPase
- pmf:
-
Proton motive force
- ROS:
-
Reactive oxygen species
- Nox:
-
NADPH oxidase
- Vrev-Hv :
-
Reversal potential of voltage-gated proton channel
- ClC7:
-
H+-Cl− antiporter of chloride channel family, ClC
- Pi:
-
Inorganic phosphate
- RANKL:
-
Receptor activator of nuclear factor κB ligand
- PKC:
-
Protein kinase C
- PMA:
-
Phorbol 12-myristate 13-acetate
References
Arai H, Berne M, Forgac M (1987) Inhibition of the coated vesicle proton pump and labeling of a 17,000 dalton polypeptide by DCCD. J Biol Chem 262:11006–11011
Arkett SA, Dixon SJ, Sims SM (1992) Substrate influences rat osteoclast morphology and expression of potassium conductances. J Physiol 458:633–653
Arkett SA, Dixon SJ, Sims SM (1994) Effects of extracellular calcium and protons on osteoclast potassium currents. J Memb Biol 140:163–171. https://doi.org/10.1007/BF00232904
Arnett TR, Dempster DW (1986) Effects of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 119:119–124. https://doi.org/10.1210/endo-119-1-119
Arnett TR, Spowage M (1996) Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 18:277–279. https://doi.org/10.1016/8756-3282(95)00486-6
Bennett BD, Alvalez U, Hruska KA (2001) Receptor-operated osteoclast calcium sensing. Endocrinology 142:1968–1974. https://doi.org/10.1210/endo.142.5.8125
Bergwitz C, Jüppner H (2011) Phosphate sensing. Adv Chronic Kidney Dis 18:132–144. https://doi.org/10.1053/j.ackd.2011.01.004
Bevington A, Kemp GJ, Graham R, Russell G (1992) Phosphate-sensitive enzymes: a possible molecular basis for cellular disorders of phosphate metabolism. Clin Chem Enzym Commun 4:235–257
Bevington A, Mundy KI, Yates AJP, Kanis JA, Russell RGG, Taylor DJ, Rajagopalan B, Radda GK (1986) A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin Sci 71:729–735. https://doi.org/10.1042/cs0710729
Blair HC, Teitelbaum SL, Ghiselli R, Gluck S (1989) Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245:855–857
Brisseau GF, Grinstein S, Hackam DJ, Nordström T, Manolson MF, Khine AA, Rotstein OD (1996) Interleukin-1 increases vacuolar-type H+-ATPase activity in murine peritoneal macrophages. J Biol Chem 271:2005–2011. https://doi.org/10.1074/jbc.271.4.2005
Brown EM, MacLeod RJ (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–297. https://doi.org/10.1152/physrev.2001.81.1.239
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359–370. https://doi.org/10.1007/s00774-015-0656-4
Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, Cherny VV, Morgan D, Gascoyne RD, Vigorito E, DeCoursey TE, MacLennan ICM, Dyer MJS (2010) HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol 11:265–272. https://doi.org/10.1038/ni.1843
Cleiren E, Bénicho O, Van Hul E, Gram J, Bollerslev J, Singer ER, Beaverson K, Aledo A, Whyte MP, Yoneyama T, deVernejoul MC, Van Hul W (2001) Albers-Schörenberg disease (autosomal dominant osteopetrosis, type II) results from mutations in CLCN7 chloride channel gene. Hum Mol Genet 10:2861–2867
Collin-Osdoby P, Osdoby P (2012) RANKL-mediated osteoclast formation from murine RAW264.7 cells. Methods Mol Biol 816:187–202. https://doi.org/10.1007/978-1-61779-415-5_13
Datta HK, MacIntyre I, Zaidi M (1989) The effect of extracellular calcium elevation on morphology and function of isolated rat osteoclasts. Biosci Rep 9:747–751. https://doi.org/10.1007/BF01114813
DeCoursey TE (2013) Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the Hv family. Physiol Rev 93:599–652. https://doi.org/10.1152/physrev.00011.2012
DeCoursey TE, Cherny VV (1998) Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J Gen Physiol 112:503–522. https://doi.org/10.1085/jgp.112.4.503
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nature Rev Mol Cell Biol 8:917–929. https://doi.org/10.1038/nrm2272
Fujita H, Matsumoto T, Kawashima H, Ogata E, Fujita T, Yamashita N (1996) Activation of Cl− channels by extracellular Ca2+ in freshly isolated rabbit osteoclasts. J Cell Physiol 169:217–225. https://doi.org/10.1002/(SICI)1097-4652(199610)169:1<217::AID-JCP22>3.0.CO;2-8
Goldhaber P, Rabadjija L (1987) H+ stimulation of cell-mediatd bone resorption in tissue culture. Am J Phys 253:E90–E98
Grano M, Faccio R, Colucci S, Paniccia R, Baldini N, Zallone AZ, Teti A (1994) Extracellular Ca2+ sensing is modulated by pH in human osteoclast-like cells in vitro. Am J Physiol 267:C961–C968. https://doi.org/10.1152/ajpcell.1994.267.4.C961
Graves AR, Curran PK, Smith CL, Mindell JA (2008) The Cl−/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453:788–792. https://doi.org/10.1038/nature06907
Gupta A, Edwards JC, Hruska KA (1996) Cellular distribution and regulation of NHE-1 isoform of the Na-H exchanger in the avian osteoclast. Bone 18:87–95. https://doi.org/10.1016/8756-3282(95)00455-6
Gupta A, Guo X-L, Alvarez UM, Hruska KA (1997) Regulation of sodium-dependent phosphate transport in osteoclasts. J Clin Invest 100:538–549. https://doi.org/10.1172/JCI119563
Gupta A, Tenenhouse HS, Hoag HM, Wang D, Khadeer MA, Namba N, Feng X, Hruska KA (2001) Identification of the type II Na+-Pi cotransporter (Npt2) in the osteoclast and the skeletal phenotype of Npt2−/− mice. Bone 29:467–476
Halleen JM, Räisänen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Väänänen HK (1999) Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartate-resistant acid phosphatase. J Biol Chem 274:22907–22910
Hammerland LG, Parihar AS, Nemeth EF, Sanguinetti MC (1994) Voltage-activated potassium currents of rabbit osteoclasts: effects of extracellular Ca2+. Am J Physiol 267:C1103–C1111. https://doi.org/10.1152/ajpcell.1994.267.4.C1103
Henderson LM, Chappell JB, Jones OTG (1988) Internal pH changes associated with the activity of NADPH oxidase of human neutrophils: further evidence for the presence of an H+ conducting channel. Biochem J 251:563–567. https://doi.org/10.1042/bj2510563
Hie M, Tsukamoto I (2011) Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur J Pharmacol 668:140–146. https://doi.org/10.1016/j.ejphar.2011.07.003
Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ (2005) The V-ATPase Vo subunit a1 is required for a late step in synaptic vesicle exocytosis in drosophila. Cell 121:607–620. https://doi.org/10.1016/j.cell.2005.03.012
Imamura H, Nakano M, Noji H, Muneyuki E, Ohkuma S, Yoshida M, Yokoyama K (2003) Evidence for rotation of V1-ATPase. Proc Natl Acad Sci U S A 100:2312–2315. https://doi.org/10.1073/pnas.0436796100
Ito M, Haito S, Furumoto M, Uehata Y, Sakurai A, Segawa H, Tatsumi S, Kuwahata M, Miyamoto K (2007) Unique uptake and efflux systems of inorganic phosphate in osteoclast-like cells. Am J Phys 292:C526–C534. https://doi.org/10.1152/ajpcell.00357.2006
Ito M, Matsuka N, Izuka M, Haito S, Sakai Y, Nakamura R, Segawa H, Kuwahata M, Yamamoto H, Pike WJ, Miyamoto K (2005) Characterization of inorganic phosphate transport in osteoclast-like cells. Am J Phys 288:C921–C931. https://doi.org/10.1152/ajpcell.00412.2004
Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S (2016) The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212:677–692. https://doi.org/10.1083/jcb.201507112
Kajiya H, Okamaoto F, Ohgi K, Nakao A, Fukushima H, Okabe K (2009) Characteristics of ClC7 Cl− channels and their inhibition in mutant (G215R) associated with autosomal dominant osteopetrosis type II in native osteoclasts and hClcn7 gene-expressing cells. Pflügers Arch 458:1049–1059. https://doi.org/10.1007/s00424-009-0689-4
Kanatani M, Sugimoto T, Kano J, Kanzawa M, Chihara K (2003) Effect of high phosphate concentration on osteoclast differentiation as well as bone resorbing activity. J Cell Physiol 196:180–189. https://doi.org/10.1002/jcp.10270
Kanaya K, Iba K, Abe Y, Dohke T, Okazaki S, Matsumura T, Yamashita T (2016) Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a bone turnover state in ovaritectomized mice. J Orthop Res 34:566–573. https://doi.org/10.1002/jor.23047. (ASIC)
Kato K, Morita I (2013) Promotion of osteoclast differentiation and activation in spite of impeded osteoclast-lineage differentiation under acidosis: effects of acidosis on bone metabolism. Biosci Trend 7:33–41. https://doi.org/10.5582/bst.2013.v7.1.33
Kelly MEM, Dixon SJ, Sims SM (1994) Outwardly rectifying chloride current in rabbit osteoclasts is activated by hyposmotic stimulation. J Physiol 475:377–389
Khadeer MA, Tang Z, Tenenhouse HS, Eiden MV, Murer H, Hernando N, Weinman EJ, Chellaiah MA, Gupta A (2003) Na+-dependent phosphate transporters in the murine osteoclast: cellular distribution and protein interactions. Am J Phys 284:C1633–C1644. https://doi.org/10.1152/ajpcell.00580.2002
Kim MS, Yang Y-M, Son A, Tian YS, Lee S-III, Kang SW, Muallem S, Shin DM (2010) RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem 285:6913–6921. https://doi.org/10.1074/jbc.M109.051557
Ko YJ, Jo WH (2010) Secondary water pore formation for proton transport in a ClC exchanger revealed by an atomic molecular dynamics stimulation. Biophys J 98:2163–2169. https://doi.org/10.1016/j.bpj.2010.01.043
Kornak U, Kasper D, Bösl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215. https://doi.org/10.1016/S0092-8674(01)00206-9
Krieger NS, Sessler NE, Bushinsky DA (1992) Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Phys 262:F442–F448. https://doi.org/10.1152/ajprenal.1992.262.3.F442
Kuno M, Ando H, Morihata H, Sakai H, Mori H, Sawada M, Oiki S (2009) Temperature dependence of proton permeation through a voltage-gated proton channel. J Gen Physiol 134:191–205. https://doi.org/10.1085/jgp.200910213
Kuno M, Kawawaki J, Nakamura F (1997) A highly temperature-sensitive proton current in mouse bone marrow-derived mast cells. J Gen Physiol 109:731–740
Kuno M, Li G, Moriura Y, Hino Y, Kawawaki J, Sakai H (2016) Acid-inducible proton influx currents in the plasma membrane of murine osteoclast-like cells. Pflűgers Archiv–Eur J Physiol 468:837–847. https://doi.org/10.1007/s00424-016-1796-7
Laitala-Leinonen T, Howell ML, Dean GE, Väänänen K (1996) Resorption-cycle-dependent polarization of mRNAs for different subunits of V-ATPase in bone-resorbing osteoclasts. Mol Biol Cell 7:129–142
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859. https://doi.org/10.1182/blood-2004-09-3662
Lee SH, Kim T, Park E-S, Yang S, Jeong D, Choi Y, Rho J (2008) NHE10, a novel osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival. Biochem Biophys Res Commun 369:320–326
Li G, Miura K, Kuno M (2017) Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflűgers Archiv–Eur J Physiol 469:279–292. https://doi.org/10.1007/s00424-016-1931-5
Li X, Xu R-S, Jiang D-L, He X-L, Jin C, Lu W-G, Su Q, Yuan F-L (2013) Acid-sensing ion channel 1a is involved in acid-induced osteoclastogenesis by regulating activation of the transcription factor NFATc1. FEBS Lett 587:3236–3242. https://doi.org/10.1016/j.febslet.2013.08.017
Longet F, Kamel S, Mentaverri R, Wattel A, Naassila M, Maamer M, Brazier M (2000) High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Comm 268:899–903. https://doi.org/10.1006/bbrc.2000.2229
Malgaroli A, Meldolesi J, Zallone AZ, Teti A (1989) Contorl of cytosolic free calcium in rat and chicken osteoclasts: the role of extracellular calcium and calcitonin. J Biol Chem 264:14342–14347
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300. https://doi.org/10.1210/er.2009-0024
Manolson MF, Yu H, Chen W, Yao Y, Li K, Lees RL, Heersche JNM (2003) The a3 isoform of the 100-kDa V-ATPase subunit is highly but differentially expressed in large (≥10 nuclei) and small (≤5 nuclei) osteoclasts. J Biol Chem 278:49271–49278. https://doi.org/10.1074/jbc.M309914200
Marshansky V, Rubinstein JL, Grüber G (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837:857–879. https://doi.org/10.1016/j.bbabio.2014.01.018
Meghji S, Morrison MS, Henderson B, Arnett TR (2001) pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am J Phys 280:E112–E119
Mentaverri A, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, Terwilliger EF, Brazier M, Brown EM (2006) The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J 20:E1945–E1954. https://doi.org/10.1096/fj.06-6304fje
Michigami T (2013) Extracellular phosphate as a signaling molecule. In: Razzaque MS (ed) Phosphate and vitamin D in chronic kidney disease. Contrib Nephrol, vol 180. Karger, Basel, pp 14–24. https://doi.org/10.1159/000346776
Miyauchi A, Hruska KA, Greenfield EM, Duncan R, Alvalez J, Barattolo R, Colucci S, Zambonin-Zallone A, Teitelbaum SL (1990) Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J Cell Biol 111:2543–2552
Moon H-J, Kim SE, Yun YP, Hwang Y-S, Bang JB, Park J-H, Kwon IK (2011) Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species. Exp Mol Med 43:605–612. https://doi.org/10.3858/emm.2011.43.11.067
Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M (2003) Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res 18:2069–2076
Mori H, Sakai H, Morihata H, Sakuta K, Kuno M (2001) A voltage gated proton channel in murine osteoclasts during development from bone marrow cells. J Bone Miner Res 16(Supple 1):S377
Mori H, Sakai H, Morihata H, Yamano T, Kuno M (2002) A voltage-gated H+ channel is a powerful mechanism for pH homeostasis in murine osteoclasts. Kobe J Med Sci 48:87–96
Mori H, Sakai H, Sakuta K, Morihata H, Kuno M (2002) Voltage-gated H+ channels and NADPH oxidases in murine osteoclasts during osteoclastogenesis. Jpn J Physiol 52(Suppl):S110
Morihata H, Nakamura F, Tsutada T, Kuno M (2000) Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J Neurosci 20:7220–7227
Mozar A, Haren N, Chasseraud M, Louvet L, Mazière C, Wattel A, Mentaverri R, Morlière P, Kamel S, Brazier M, Mazière JC, Massy ZA (2008) High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J Cell Physiol 215:47–54. https://doi.org/10.1002/jcp.21283
Nakamura I, Sasaki T, Tanaka S, Takahashi N, Jimi E, Kurokawa T, Kita Y, Ihara S, Suda T, Fukui Y (1997) Phosphatidylinositol-3 kinase is involved in ruffled border formation in osteoclasts. J Cell Physiol 172:230–239. https://doi.org/10.1002/(SICI)1097-4652(199708)172:2<230::AID-JCP10>3.0.CO;2-D
Nelson N, Harvey WR (1999) Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79:361–385. https://doi.org/10.1152/physrev.1999.79.2.361
Nesbitt SA, Horton MA (1997) Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 276:266–269
Nordström T, Rotstein OD, Romanek R, Asotra S, Heersche JNM, Manolson MF, Brisseau GF, Grinstein S (1995) Regulation of cytoplasmic pH in osteoclasts: contribution of proton pumps and a proton-selective conductance. J Biol Chem 270:2203–3312
Nordström T, Shrode LD, Rotstein OD, Romanek R, Goto T, Heersche JNM, Manolson MF, Brisseau GF, Grinstein S (1997) Chronic extracellular acidosis induces plasmalemmal vacuolar type H+ ATPase activity in osteoclasts. J Biol Chem 272:6354–6360. https://doi.org/10.1074/jbc.272.10.6354
Notomi T, Kuno M, Hiyama A, Ohura K, Noda M, Skerry TM (2015) Zinc-induced effects on osteoclastogenesis involve activation of hyperpolarization-activated cyclic nucleotide modulated channels via changes in membrane potential. J Bone Miner Res 30:1618–1626. https://doi.org/10.1002/jbmr.2507
Okochi Y, Sasaki M, Iwasaki H, Okamura Y (2009) Voltage-gated proton channels is expressed on phagosomes. Biochem Biophys Res Comm 382:274–279. https://doi.org/10.1016/j.bbrc.2009.03.036
Pantazis A, Keegan P, Postma M, Schwiening CJ (2006) The effect of neuronal morphology and membrane-permeant weak acid and base on the dissipation of depolarization-induced pH gradients in snail neurons. Pflugers Arch–Eur J Physiol 452:175–187. https://doi.org/10.1007/s00424-005-0019-4
Park KH, Park B, Yoon DS, Kwon S-H, Shin DM, Lee JW, Lee HG, Shim J-H, Park JH, Lee JM (2013) Zinc inhibits osteoclast differentiation by suppression of Ca2+-calcineurin-NFATc1 signaling pathway. Cell Comm Signal 11:74. https://doi.org/10.1186/1478-811X-11-74
Peters C, Bayer MJ, Bühler S, Andersen JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588. https://doi.org/10.1038/35054500
Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209. https://doi.org/10.1074/jbc.M505186200
Ramsey IS, Moran MM, Chong JA, Clapham DE (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440:1213–1216. https://doi.org/10.1038/nature04700
Ravesloot JH, Eisen T, Baron R, Boron WF (1995) Role of Na-H exchangers and vacuolar H+ pumps in intracellular pH regulation in neonatal rat osteoclasts. J Gen Physiol 105:177–208
Recchi C (2006) Chavrier P (2006) V-ATPase: a potential pH sensor. Nat Cell Biol 8:107–109. https://doi.org/10.1038/ncb0206-107
Sakai H, Kawawaki J, Moriura Y, Mori H, Morihata H, Kuno M (2006) pH dependence and inhibition by extracellular calcium of proton currents via plasmalemmal vacuolar-type H+-ATPase in murine osteoclasts. J Physiol 576(2):417–425. https://doi.org/10.1113/physiol.2006.117176
Sakai H, Li G, Hino Y, Moriura Y, Kawawaki J, Sawada M, Kuno M (2013) Increases in intracellular pH facilitate endocytosis and decrease availability of voltage-gated proton channels in osteoclasts and microglia. J Physiol 591(23):5851–5866. https://doi.org/10.1113/jphysiol.2013.263558
Sakai H, Moriura Y, Kawawaki J, Hashimoto S, Kuno M (2011) Vacuolar H+-ATPases and voltage-gated proton channels: two electrogenic, proton-selective membrane transport mechanisms co-existed in osteoclasts. Biophys J 100(supple):93
Sakai H, Moriura Y, Notomi T, Kawawaki J, Ohnishi K, Kuno M (2010) Phospholipase C-dependent Ca2+-sensing pathways leading to endocytosis and inhibition of the plasma membrane vacuolar H+-ATPase in osteoclasts. Am J Phys 299:C570–C578. https://doi.org/10.1152/ajpcell.00486.2009
Sakai H, Nakamura F, Kuno M (1999) Synergetic activation of outwardly rectifying Cl− currents by hypotonic stress and external Ca2+ in murine osteoclasts. J Physiol 515:157–168
Sakuta K, Sakai H, Mori H, Morihata H, Kuno M (2002) Na+-dependence of extracellular calcium-sensing mechanisms leading to activation of an outwardly rectifying Cl− channel in murine osteoclasts. Bone 31:374–380
Sasaki M, Takagi M, Okamura Y (2006) A voltage sensor-domain protein is a voltage-gated proton channel. Science 312:589–592. https://doi.org/10.1126/science.1122352
Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K (2009) NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Investig 56:33–41
Schlesinger PH, Blair HC, Teitelbaum SL, Edwards JC (1994) Characterization of the osteoclast ruffled border chloride channel and its role in bone resorption. J Biol Chem 272:18636–18643
Schröder K (2015) NADPH oxidases in bone homeostasis and osteoporosis. Cell Mol Life Sci 72:25–38. https://doi.org/10.1007/s00018-014-1712-2
Shibata T, Sakai H, Nakamura F, Shioi A, Kuno M (1997) Differential effect of high extracellular Ca2+ on K+ and Cl− conductances in murine osteoclasts. J Memb Biol 158:59–67
Silver IA, Nurrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175:266–276
Sims SM, Dixon SJ (1989) Inwardly rectifying K+ current in osteoclasts. Am J Phys 256:C1277–C1282. https://doi.org/10.1152/ajpcell.1989.256.6.C1277
Sly WS, Hewett-Emmett D, Whyte MP, Yu YSL, Tashian RE (1983) Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 80:2752–2756
Spitzer KW, Ershler PR, Skolnick RL, Vaughan-Jones RD (2000) Generation of intracellular pH gradients in single cardia myocytes with a microperfusion system. Am J Phys 278:H1371–H1382. https://doi.org/10.1152/ajpheart.2000.278.4.H1371
Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savariayan R, Villa A, Orchard P, Vezzoni P, Albertini A, Frattini A, Pagani F (2004) TCIRG1-deficient recessive osteopetrosis: mutation analysis, functional identification of the splicing defects, and in vitro rescue by U1 snRNA. Hum Mutat 24:225–235. https://doi.org/10.1002/humu.20076
Swallow CJ, Grinstein S, Sudsbury RA, Rotstein OD (1993) Relative roles of Na+/H+ exchange and vacuolar type H+-ATPases in regulating cytoplasmic pH and function in murine peritoneal macrophages. J Cell Physiol 157:453–460. https://doi.org/10.1002/jcp.1041570304
Tanaka S (2013) Regulation of bone destruction in rheumatoid arthritis through RANKL-RANK pathways. World J Orthop 18:1–6. https://doi.org/10.5312/wjo.v4.i1.1
Teti A, Blair HC, Schlesinger P, Grano M, Zambonin-Zallone A, Kahn AJ, Teitelbaum SL, Hruska KA (1989) Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. J Clin Invest 84:773–780. https://doi.org/10.1172/JCI114235
Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada G, Wada Y, Futai M (2003) From lysosomes to the plasma membrane: localization of vacuolar type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278:22023–22030. https://doi.org/10.1074/jbc.M302436200
Väänänen HK, Karhukorpi EK, Sundquist K, Wallmark B, Roininen I, Hentunen T, Tukkanen J, Lakkakorpi P (1990) Evidence for the presence of a proton pump of the vacuolar H+-ATPase type in the ruffled borders of osteoclasts. J Cell Biol 111:1305–1311
Xu J, Feng HT, Wang C, Yip KHM, Pavlos N, Papadimitriou JM, Wood D, Zheng MH (2003) Effects of bafilomycin A1: an inhibitor of vacuolar H+-ATPases on endocytosis and apoptosis in RAW cells and raw cell-derived osteoclasts. J Cell Biochem 88:1256–1264. https://doi.org/10.1002/jcb.10477
Yabe I, Horiuchi K, Nakahara K, Hiyama T, Yamanaka T, Wang P-C, Toda K, Hirata A, Ohsumi Y, Hirata R, Anraku Y, Kusaka I (1999) Patch clamp studies on V-type ATPase of vacuolar membrane of haploid saccharomyces cerevisiae: preparation and utilization of a giant cell containing a giant vacuole. J Biol Chem 274:34903–34910
Yamashita N, Ishii T, Ogata E, Mastumoto T (1994) Inhibition of inwardly rectifying K+ currents by external Ca2+ ions in freshly isolated rabbit osteoclasts. J Physiol 480:217–224
Yates AJ, Oreffo ROC, Mayor K, Mundy GR (1991) Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J Bone Miner Res 6:473–478. https://doi.org/10.1002/jbmr.5650060508
Yokoyama K, Nakano M, Imamura H, Yoshida M, Tamakoshi M (2003) Rotation of the proteolipid ring in the V-ATPase. J Biol Chem 278:24255–24258. https://doi.org/10.1074/jbc.M303104200
Yoneda T, Hiasa M, Nagata Y, Okui T, White F (2015) Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain. Biochim Biophys Acta 1848:2677–2684. https://doi.org/10.1016/j.bbamem.2015.02.004
Zaidi M, Alam AS, Huang CL, Pazianas M, Bax CM, Bax BL, Moonga BS, Bevis PJ, Shankar VS (1993) Extracellular Ca2+ sensing by the osteoclast. Cell Calcium 14:271–277. https://doi.org/10.1016/0143-4160(93)90048-B
Zaidi M, Kerby J, Huang CL, Alam T, Rathod H, Chambers TJ, Moonga BS (1991) Divalent cations mimic the inhibitory effect of extracellular ionized calcium on bone resorption by isolated rat osteoclasts: further evidence for a “calcium receptor”. J Cell Physiol 149:422–427. https://doi.org/10.1002/jcp.1041490310
Zhang W, Wang D, Volk E, Bellen HJ, hiesinger PR, Quiocho FA (2008) V-ATPase Vo sector subunit a1 in neurons is a target of calmodulin. J Biol Chem 283:294–300. https://doi.org/10.1074/jbc.M708058200
Acknowledgements
I thank Drs. Hiromu Sakai, Fusao Nakamura, Guangshuai Li, Hirokazu Morihata, Hiroyuki Mori, Takuya Notomi, Koichi Sakuta, Toshiya Shibata for collaboration, Junko Kawawaki, Yoshie Moriura and Yoshiko Hino for technical assistance, and Dr. Charles Edwards for critically reading our manuscripts. This work was supported by JSPS KAKENHI Grants (JP15K08184).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest.
Rights and permissions
About this article
Cite this article
Kuno, M. Cooperative electrogenic proton transport pathways in the plasma membrane of the proton-secreting osteoclast. Pflugers Arch - Eur J Physiol 470, 851–866 (2018). https://doi.org/10.1007/s00424-018-2137-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2137-9