Abstract
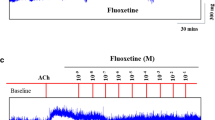
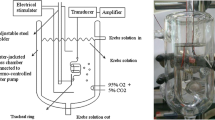
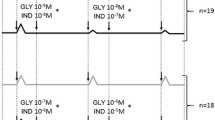
This study aimed at investigating the potential ghrelin relaxing effect on guinea pig isolated tracheal smooth muscle (TSM). Using an in vitro experimental approach, the physiological role of the airway epithelium on smooth muscle relaxation has been investigated by analyzing the dose-response curves for carbachol- or histamine-induced contractions on epithelium intact versus denuded tracheal tissue. The relaxant effect of ghrelin (5–200 μmol/L) then investigated on carbachol-contracted, non-sensitized, and ovalbumin (OVA)-sensitized guinea pig TSM with an intact or denuded epithelium. The isolated TSMs from identical guinea pigs were incubated in Krebs solution aerated with 95% O2 and 5% CO2 through an automated tissue organ bath system (n = 6 for each group). The ghrelin relaxation mechanism was assessed by adding L-NAME, indomethacin, and YIL-781 for GHS-R1 into the tissue chamber. The spasmogens carbachol and histamine have shown a significantly higher contracting effect on epithelium-denuded than in epithelium-intact TSM confirmed by the significantly higher mean pEC50 of both agonists on the epithelium-denuded trachea (p < 0.05). Ghrelin has shown a concentration-dependent relaxing effect on carbachol-contracted TSM (r = 0.96, p = 0.00). The effect was more evident in the intact non-sensitized than in epithelium-denuded or OVA-sensitized groups (p < 0.05). Preincubation with nitric oxide (NO) and prostaglandin E2 (PGE2) inhibitors has significantly reduced the ghrelin-induced relaxation on epithelium-intact TSM suggesting an epithelium-dependant mechanism. However, GHS-R1a antagonist has also succeeded to reduce ghrelin relaxant effect, which needs further clarification. Ghrelin proved to have a potential TSM relaxant effect possibly through epithelium-dependant mechanisms involving NO and PGE2.









Similar content being viewed by others
References
Baatar D, Patel K, Taub DD (2011) The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 340:44–58
Bedendi I, Alloatti G, Marcantoni A, Malan D, Catapano F, Ghe C et al (2003) Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. Eur J Pharmacol 476:87–95
Boskabady MH, Khatami A, Nazari A (2004) Possible mechanism(s) for relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. Pharmazie 59:561–564
Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, Longrois D, Norel X (2012) PGE(2) receptor (EP(4)) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther 25(1):115–118
Bano S, Swati O, Kambadur M, Mohammad F (2016) Deterioration of epithelium mediated mechanisms in diabetic-antigen sensitized airways of guinea pigs. J Smooth Muscle Res 52(0):93–104
Benoit C, Renaudon B, Salvail D, Rousseau E (2001) EETs relax airway smooth muscle via an EpDHF effect: BK (Ca) channel activation and hyperpolarization. Am J Physiol Lung Cell Mol Physiol 280(5):L965–L973
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. JClin Invest 117:13–23
Carsin A, Mazenq J, Ilstad A, Dubus JC, Chanez P, Gras D (2016) Bronchial epithelium in children: a key player in asthma. EurRespir Rev 25(140):158–169
Campbell WB, Fleming I (2010) Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459(6):881–895
McCaig DJ (1987) Comparison of autonomic responses in the trachea isolated from normal and albumin-sensitive guinea-pigs. Br J Pharmaco l92(4):809–816
Fu T, Wang L, Zeng Q, Zhang Y, Sheng B, Han L (2017) Ghrelin ameliorates asthma by inhibiting endoplasmic reticulum stress. Am J Med Sci 354(6):617–625
Govindan S, Taylor CW (2012) P2Y receptor subtypes evoke different Ca2+ signals in cultured aortic smooth muscle cells. Purinergic Signal 8(4):763–777
Hassouna R, Labarthe A, Tolle V (2016) Hypothalamic regulation of body growth and appetite by ghrelin-derived peptides during balanced nutrition or undernutrition. Mol Cell Endocrinol 438:42–51
Hehir MP, Glavey SV, Morrison JJ (2008) Uterorelaxant effect of ghrelin on human myometrial contractility. Am. J. Obstet. Gynec 198(3):323.e1–323.e5
Herrerias A, Torres R, Serra M, Marco A, Pujols L, Picado C, de Mora F (2009) Activity of the cyclooxygenase 2 prostaglandin-E prostanoid receptor pathway in mice exposed to house dust mite aeroallergens, and impact of exogenous prostaglandin E2. J Inflamm (Lond) 30(6):30
Iantorno M, Chen H, Kim JA, Tesauro M, Lauro D, Cardillo C, Quon MJ (2007) Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. Am J Physiol Endocrinol Metab 292(3):E756–E764
Kirchner H, Heppner KM, Tschop MH (2012) The role of ghrelin in the control of energy balance. Handbook of experimental pharmacology 161–184.
Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M (2008) Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther 21(5):774–779
Lago F, Gonzalez-Juanatey JR, Casanueva FF, Gomez-Reino J, Dieguez C, Gualillo O (2005) Ghrelin, the same peptide for different functions: player or bystander? Vitam Horm 71:405–432
Luo FM, Liu XJ, Li SQ, Wang ZL, Liu CT, Yuan YM (2005) Circulating ghrelin in patients with chronic obstructive pulmonary disease. Nutrition:793–798
Matsuda K, Nishi Y, Okamatsu Y, Kojima M, Matsuishi T (2006) Ghrelin and leptin: a link between obesity and allergy? J Allergy Clin Immunol 117(3):705–706
Maniscalco M, Sofia M, Pelaia G (2007) Nitric oxide in upper airways inflammatory diseases. Inflamm Res 56(2):58–69
Morin C, Proteau S, Rousseau E, Brayden J (2005) Organ-cultured airway explants: a new model of airway hyperresponsiveness. Exp Lung Res 31(7):719–744
Morin C, Rousseau E (2007) Effects of 5-oxo-ETE and 14,15-EET on reactivity and Ca2+ sensitivity in guinea pig bronchi. Prostaglandins Other Lipid Mediat 82(1–4):30–41 Epub 2006 Jul 24
Merigo F, Boschi F, Lasconi C, Benati D, Sbarbati A (2016) Molecules implicated in glucose homeostasis are differentially expressed in the trachea of lean and obese Zucker rats. Eur J Histochem 60(1):2557
Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K (2002) Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J Cardiovasc Pharmacol 39(6):779–783
Peng M, Cai BQ, Ma Y, Zhu HJ, Sun Q, Song AL (2007) Circulating leptin and ghrelin in patients with chronic obstructive pulmonary disease. ZhonghuaJie He He Hu Xi ZaZhi 30(3):182–185
Plant PJ, North ML, Ward A, Ward M, Khanna N, Correa J, Scott JA, Batt J (2012) Hypertrophic airway smooth muscle mass correlates with increased airway responsiveness in a murine model of asthma. Am J Respir Cell Mol Biol 46(4):532–540
Penn RB, Benovic JL (2008) Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am ThoracSoc 5(1):47–57
Pavlovic D, Frieling H, Usichenko T, Nedeljkov V, Nafissi T, Lehmann C et al (2008) S-carboxymethylcysteine inhibits carbacholinduced constriction of epithelium-denuded rat and human airway preparations. ClinExpPharmacolPhysiol 35:663–669
Rocha-Sousa A, Saraiva J, Henriques-Coelho T, Falcão-Reis F, Correia-Pinto J, Leite-Moreira AF (2006) Ghrelin as a novel locally produced relaxing peptide of the iris sphincter and dilator muscles. Exp Eye Res 83(5):1179–1187
Ricciardolo FL, Nijkamp F, De Rose V, Folkerts G (2008) The guinea pig as an animal model for asthma. Curr Drug Targets 9(6):452–465
Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M (2012) Structure, regulation and function of ghrelin. J Biochem 151:119–128
Suzuki K, Jayasena CN, Bloom SR (2012) Obesity and appetite control. Exp Diabetes Res Article ID 824305, 19 pagesdoi:10.1155/2012/824305.
Souza-Moreira L, Campos-Salinas J, Caro M, Gonzalez-Rey E (2011) Neuropeptides as pleiotropic modulators of the immune response. Neuroendocrinology 94(2):89–100
Sahin G, Klimek L, Mullol J, Hörman K, Walther LE, Pfaar O (2011) Nitric oxide: a promising methodological approach in airway diseases. Int Arch Allergy Immunol1 56(4):352–361
Semenov I, Herlihy JT, Brenner R (2012) In vitro measurements of tracheal constriction using mice. J Vis Exp 25(64):3703
Scott GD, Fryer AD (2012) Role of parasympathetic nerves and muscarinic receptors in allergy and asthma. Chem Immunol Allergy 98:48–69
Toru Ü, Ayada C, Genç O, Şahin S, Arık Ö, Acat M, Bulut İ, Çetinkaya E (2015) Visfatin and ghrelin: can they be forthcoming biomarkers or new drug targets for asthma? Int J ClinExp Med 8(4):6257–6261 (High serum ghrelin in asthmatics—anti-inflammatory)
Varela L, Vazquez MJ, Cordido F, Nogueiras R, Vidal-Puig A, Dieguez C et al (2011) Ghrelin and lipid metabolism: key partners in energy balance. J Mol Endocrinol 46:R43–R63
Volante M, Fulcheri E, Allìa E, Cerrato M, Pucci A, Papotti M (2002) Ghrelin expression in fetal, infant, and adult human lung. J Histochem Cytochem 50:1013–1021
Zhou J, Alvarez-Elizindo MB, Botvinick E, George SC (2012) Local small airway epithelial injury induces global smooth muscle contraction and airway constriction. J Appl Physiol 112:627–637
Acknowledgements
I would like to acknowledge Dr. Aamir Magzoub & Dr. AdilWaddad (College of Medicine), Mr. Ibrahim Shaikh & Mr. Mohammed ShafiuddinHabeeb (College of Pharmacy), Najran University for their valuable assistance in this work.
Funding
This study was supported by King Abdulaziz City for Science and Technology (KACST) for funding this project with No. A.T.34/116.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study was ethically approved by the research ethics committee at the College of Medicine, Najran University, Kingdom of Saudi Arabia.
Rights and permissions
About this article
Cite this article
Al-Ayed, M.S.Z. Relaxant effect of ghrelin on guinea pig isolated tracheal smooth muscle: role of epithelial NO and PGE2. Pflugers Arch - Eur J Physiol 470, 949–958 (2018). https://doi.org/10.1007/s00424-018-2126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2126-z