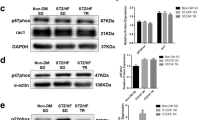
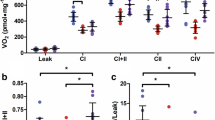
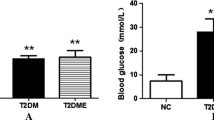
Abstract
Metabolic disturbance and mitochondrial dysfunction are a hallmark of diabetic cardiomyopathy (DC). Resistance exercise (RE) not only enhances the condition of healthy individuals but could also improve the status of those with disease. However, the beneficial effects of RE in the prevention of DC and mitochondrial dysfunction are uncertain. Therefore, this study investigated whether RE attenuates DC by improving mitochondrial function using an in vivo rat model of diabetes. Fourteen Otsuka Long-Evans Tokushima Fatty rats were assigned to sedentary control (SC, n = 7) and RE (n = 7) groups at 28 weeks of age. Long-Evans Tokushima Otsuka rats were used as the non-diabetic control. The RE rats were trained by 20 repetitions of climbing a ladder 5 days per week. RE rats exhibited higher glucose uptake and lower lipid profiles, indicating changes in energy metabolism. RE rats significantly increased the ejection fraction and fractional shortening compared with the SC rats. Isolated mitochondria in RE rats showed increase in mitochondrial numbers, which were accompanied by higher expression of mitochondrial biogenesis proteins such as proliferator-activated receptor-γ coactivator-1α and TFAM. Moreover, RE rats reduced proton leakage and reactive oxygen species production, with higher membrane potential. These results were accompanied by higher superoxide dismutase 2 and lower uncoupling protein 2 (UCP2) and UCP3 levels in RE rats. These data suggest that RE is effective at ameliorating DC by improving mitochondrial function, which may contribute to the maintenance of diabetic cardiac contractility.





Similar content being viewed by others
References
Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106:1253–1264. https://doi.org/10.1161/CIRCRESAHA.109.213116
An D, Rodrigues B (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 291:H1489–H1506. https://doi.org/10.1152/ajpheart.00278.2006
Ashford AJ, Pain VM (1986) Effect of diabetes on the rates of synthesis and degradation of ribosomes in rat muscle and liver in vivo. J Biol Chem 261:4059–4065
Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA (2010) Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech 7:e135–e143. https://doi.org/10.1016/j.ddmec.2010.08.001
Benard G, Rossignol R (2008) Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal 10:1313–1342. https://doi.org/10.1089/ars.2007.2000
Bosetti F, Baracca A, Lenaz G, Solaini G (2004) Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett 563:161–164. https://doi.org/10.1016/S0014-5793(04)00294-7
Brahma MK, Pepin ME, Wende AR (2017) My sweetheart is broken: role of glucose in diabetic cardiomyopathy. Diabetes Metab J 41:1–9. https://doi.org/10.4093/dmj.2017.41.1.1
Braith RW, Stewart KJ (2006) Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation 113:2642–2650. https://doi.org/10.1161/CIRCULATIONAHA.105.584060
Burri L, Thoresen GH, Berge RK (2010) The role of PPARalpha activation in liver and muscle. PPAR Res 2010. https://doi.org/10.1155/2010/542359
Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P (2005) The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 86:1527–1533. https://doi.org/10.1016/j.apmr.2005.01.007
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008) Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol (1985) 104:371–378. https://doi.org/10.1152/japplphysiol.00873.2007
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813:1351–1359. https://doi.org/10.1016/j.bbamcr.2011.01.014
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415:96–99. https://doi.org/10.1038/415096a
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 29:270–276. https://doi.org/10.1093/eurheartj/ehm342
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, Anderson JL (2012) 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 126:e354–e471. https://doi.org/10.1161/CIR.0b013e318277d6a0
Fruchart JC, Staels B, Duriez P (2001) The role of fibric acids in atherosclerosis. Curr Atheroscler Rep 3:83–92
Gaborit B, Kober F, Jacquier A, Moro PJ, Cuisset T, Boullu S, Dadoun F, Alessi MC, Morange P, Clement K, Bernard M, Dutour A (2012) Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes 36:422–430. https://doi.org/10.1038/ijo.2011.117
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:117. https://doi.org/10.1186/gb-2003-4-9-117
Guelfi KJ, Jones TW, Fournier PA (2005) The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 28:1289–1294
Ha MW, Ma R, Shun LP, Gong YH, Yuan Y (2005) Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol 11:5433–5437
Hare JL, Hordern MD, Leano R, Stanton T, Prins JB, Marwick TH (2011) Application of an exercise intervention on the evolution of diastolic dysfunction in patients with diabetes mellitus: efficacy and effectiveness. Circ Heart Fail 4:441–449. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959312
Jahng JW, Song E, Sweeney G (2016) Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med 48:e217. https://doi.org/10.1038/emm.2016.20
Jeong SH, Kim HK, Song IS, Lee SJ, Ko KS, Rhee BD, Kim N, Mishchenko NP, Fedoryev SA, Stonik VA, Han J (2014) Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar Drugs 12:2922–2936. https://doi.org/10.3390/md12052922
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34:29–34
Kim HK, Youm JB, Lee SR, Lim SE, Lee SY, Ko TH, Long le T, Nilius B, Won du N, Noh JH, Ko KS, Rhee BD, Kim N, Han J (2012) The angiotensin receptor blocker and PPAR-gamma agonist, telmisartan, delays inactivation of voltage-gated sodium channel in rat heart: novel mechanism of drug action. Pflugers Arch 464:631–643. https://doi.org/10.1007/s00424-012-1170-3
Kim JY, Choi MJ, So B, Kim HJ, Seong JK, Song W (2015) The preventive effects of 8 weeks of resistance training on glucose tolerance and muscle fiber type composition in Zucker rats. Diabetes Metab J 39:424–433. https://doi.org/10.4093/dmj.2015.39.5.424
Lago RM, Singh PP, Nesto RW (2007) Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370:1129–1136. https://doi.org/10.1016/S0140-6736(07)61514-1
Larsen MO, Rolin B, Wilken M, Carr RD, Gotfredsen CF (2003) Measurements of insulin secretory capacity and glucose tolerance to predict pancreatic beta-cell mass in vivo in the nicotinamide/streptozotocin Gottingen minipig, a model of moderate insulin deficiency and diabetes. Diabetes 52:118–123
Lee TW, Bai KJ, Lee TI, Chao TF, Kao YH, Chen YJ (2017) PPARs modulate cardiac metabolism and mitochondrial function in diabetes. J Biomed Sci 24:5. https://doi.org/10.1186/s12929-016-0309-5
Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ (2013) Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 14:585–592. https://doi.org/10.1016/j.jamda.2013.02.006
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. https://doi.org/10.1371/journal.pbio.0030101
Ma X, Fu Y, Xiao H, Song Y, Chen R, Shen J, An X, Shen Q, Li Z, Zhang Y (2015) Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One 10:e0129971. https://doi.org/10.1371/journal.pone.0129971
Mann N, Rosenzweig A (2012) Can exercise teach us how to treat heart disease? Circulation 126:2625–2635. https://doi.org/10.1161/CIRCULATIONAHA.111.060376
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik K, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A (2012) [ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012]. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir 40 Suppl 3:77–137
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166. https://doi.org/10.1007/s10741-012-9313-3
Moreno-Santos I, Perez-Belmonte LM, Macias-Gonzalez M, Mataro MJ, Castellano D, Lopez-Garrido M, Porras-Martin C, Sanchez-Fernandez PL, Gomez-Doblas JJ, Cardona F, de Teresa-Galvan E, Jimenez-Navarro M (2016) Type 2 diabetes is associated with decreased PGC1alpha expression in epicardial adipose tissue of patients with coronary artery disease. J Transl Med 14:243. https://doi.org/10.1186/s12967-016-0999-1
Motoori S, Majima HJ, Ebara M, Kato H, Hirai F, Kakinuma S, Yamaguchi C, Ozawa T, Nagano T, Tsujii H, Saisho H (2001) Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res 61:5382–5388
Mujika I, Padilla S (2000) Detraining: loss of training-induced physiological and performance adaptations. Part II: long term insufficient training stimulus. Sports Med 30:145–154
Naderi R, Mohaddes G, Mohammadi M, Ghaznavi R, Ghyasi R, Vatankhah AM (2015) Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv Pharm Bull 5:231–236. 10.15171/apb.2015.032
Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB (2015) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47:1922–1931. https://doi.org/10.1249/MSS.0000000000000605
Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V (2013) Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol 1:86–93. https://doi.org/10.1016/j.redox.2012.11.009
Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M (2009) Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 54:1524–1532. https://doi.org/10.1016/j.jacc.2009.04.074
Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z (2012) PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One 7:e41817. https://doi.org/10.1371/journal.pone.0041817
Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA (2011) Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 286:10605–10617. https://doi.org/10.1074/jbc.M110.211466
Schupp M, Kintscher U, Fielitz J, Thomas J, Pregla R, Hetzer R, Unger T, Regitz-Zagrosek V (2006) Cardiac PPARalpha expression in patients with dilated cardiomyopathy. Eur J Heart Fail 8:290–294. https://doi.org/10.1016/j.ejheart.2005.09.003
Selig SE, Carey MF, Menzies DG, Patterson J, Geerling RH, Williams AD, Bamroongsuk V, Toia D, Krum H, Hare DL (2004) Moderate-intensity resistance exercise training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Card Fail 10:21–30
Seo DY, Lee S, Figueroa A, Kwak YS, Kim N, Rhee BD, Ko KS, Bang HS, Baek YH, Han J (2012) Aged garlic extract enhances exercise-mediated improvement of metabolic parameters in high fat diet-induced obese rats. Nutr Res Pract 6:513–519. https://doi.org/10.4162/nrp.2012.6.6.513
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2014) Humanized animal exercise model for clinical implication. Pflugers Arch 466:1673–1687. https://doi.org/10.1007/s00424-014-1496-0
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2016) Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res 5:182–186. https://doi.org/10.1016/j.imr.2016.07.003
Seo DY, Lee SR, Kwak HB, Seo KW, McGregor RA, Yeo JY, Ko TH, Bolorerdene S, Kim N, Ko KS, Rhee BD, Han J (2016) Voluntary stand-up physical activity enhances endurance exercise capacity in rats. Korean J Physiol Pharmacol 20:287–295. https://doi.org/10.4196/kjpp.2016.20.3.287
Shim CY, Song BW, Cha MJ, Hwang KC, Park S, Hong GR, Kang SM, Lee JE, Ha JW, Chung N (2014) Combination of a peroxisome proliferator-activated receptor-gamma agonist and an angiotensin II receptor blocker attenuates myocardial fibrosis and dysfunction in type 2 diabetic rats. J Diabetes Investig 5:362–371. https://doi.org/10.1111/jdi.12153
Shim IK, Cho KI, Kim HS, Heo JH, Cha TJ (2015) Impact of gender on the association of epicardial fat thickness, obesity, and circadian blood pressure pattern in hypertensive patients. J Diabetes Res 2015:924539. https://doi.org/10.1155/2015/924539
Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, Hesselink MK, Smith SR, Schrauwen P (2013) Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab 98:1694–1702. https://doi.org/10.1210/jc.2012-3874
Suh S, Seo GH, Jung CH, Kim MK, Jin SM, Hwang YC, Lee BW, Kim JH (2015) Increased risk of hospitalization for heart failure with newly prescribed dipeptidyl peptidase-4 inhibitors and pioglitazone using the Korean Health Insurance Claims Database. Diabetes Metab J 39:247–252. https://doi.org/10.4093/dmj.2015.39.3.247
Thu VT, Kim HK, Long le T, Lee SR, Hanh TM, Ko TH, Heo HJ, Kim N, Kim SH, Ko KS, Rhee BD, Han J (2012) NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res 94:342–350. https://doi.org/10.1093/cvr/cvs122
Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC (2016) Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol 92:163–173. https://doi.org/10.1016/j.yjmcc.2016.01.023
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. https://doi.org/10.1038/nrg3185
Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, Cao Y, Chen P, Xiao J, Kong X (2015) Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1alpha and Akt activation. Cell Physiol Biochem 35:2159–2168. https://doi.org/10.1159/000374021
Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH (2009) Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 23:1603–1612. https://doi.org/10.1210/me.2009-0033
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:1810–1852. https://doi.org/10.1161/CIR.0b013e31829e8807
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87:320–323
Funding
This work was supported by the grant from the Priority Research Centers Program and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2010-0020224, NRF-2015R1A2A1A13001900) and Korean Diabetes Association (J.C.W., 2015F2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures were approved by the Institutional Review Board of Animals, Inje University College of Medicine (approval number: 2011-049).
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Ko, T.H., Marquez, J.C., Kim, H.K. et al. Resistance exercise improves cardiac function and mitochondrial efficiency in diabetic rat hearts. Pflugers Arch - Eur J Physiol 470, 263–275 (2018). https://doi.org/10.1007/s00424-017-2076-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2076-x