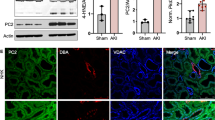
Abstract
Although autosomal dominant polycystic kidney disease (ADPKD) is characterized by the development of multiple kidney cysts, the most frequent cause of death in ADPKD patients is cardiovascular disease. ADPKD is linked to mutations in PKD1 or pkd2, the genes that encode for the proteins polycystin 1 and polycystin 2 (PC1 and PC2, respectively). The cardiovascular complications have been assumed to be a consequence of renal hypertension and activation of renin/angiotensin/aldosterone (RAAS) pathway. However, the expression of PC1 and PC2 in cardiac tissue suggests additional direct effects of these proteins on cardiac function. We previously reported that zebrafish lacking PC2 develop heart failure, and that heterozygous Pkd2+/− mice are hypersensitive to acute β-adrenergic receptor (βAR) stimulation. Here, we investigate the effect of cardiac stress (prolonged continuous βAR stimulus) on Pkd2+/− mice. After βAR stimulation for 7 days, wild-type (WT) mice had increased left ventricular mass and natriuretic peptide (ANP and BNP) mRNA levels. The WT mice also had upregulated levels of PC2 and chromogranin B (CGB, an upstream regulator of BNP). Conversely, Pkd2+/− mice had increased left ventricular mass, but natriuretic peptide and CGB expression levels remained constant. Reversal of the increased cardiac mass was observed in WT mice 3 days after cessation of the βAR stimulation, but not in Pkd2+/− mice. We suggest that cardiac stress leads to upregulation of the PC2-CGB-BNP signaling axis, and this pathway regulates the production of cardio-protective natriuretic peptides. The lack of a PC2-dependent cardio-protective function may contribute to the severity of cardiac dysfunction in Pkd2+/− mice and in ADPKD patients.






Similar content being viewed by others
References
Alam A, Perrone RD (2013) Left ventricular hypertrophy in ADPKD: changing demographics. Curr Hypertens Rev 9:27–31
Anyatonwu GI, Estrada M, Tian X et al (2007) Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A 104:6454–6459
Azibani F, Benard L, Schlossarek S et al (2012) Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension 59:1179–1187
Bardaji A, Vea AM, Gutierrez C et al (1998) Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis 32:970–975
Barrett BJ, Foley R, Morgan J et al (1994) Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int 46:1118–1123
Chapman AB, Johnson AM, Rainguet S et al (1997) Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8:1292–1297
Clerico A, Giannoni A, Vittorini S et al (2011) Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol 301:H12–H20
Fick GM, Johnson AM, Hammond WS et al (1995) Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5:2048–2056
Friddle CJ, Koga T, Rubin EM et al (2000) Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proc Natl Acad Sci U S A 97:6745–6750
Harrap SB, Davies DL, Macnicol AM et al (1991) Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int 40:501–508
Harris PC, Torres VE (2014) Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124:2315–2324
Heidrich FM, Zhang K, Estrada M et al (2008) Chromogranin B regulates calcium signaling, nuclear factor kappaB activity, and brain natriuretic peptide production in cardiomyocytes. Circ Res 102:1230–1238
Heyer CM, Sundsbak JL, Abebe KZ et al (2016) Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27:2872–2884
Hohimer AR, Davis LE, Hatton DC (2005) Repeated daily injections and osmotic pump infusion of isoproterenol cause similar increases in cardiac mass but have different effects on blood pressure. Can J Physiol Pharmacol 83:191–197
Holditch SJ, Schreiber CA, Nini R et al (2015) B-type natriuretic peptide deletion leads to progressive hypertension, associated organ damage, and reduced survival: novel model for human hypertension. Hypertension (Dallas, Tex. : 1979) 66:199–210
Huang Y, Di Lorenzo A, Jiang W et al (2013) Hypoxia-inducible factor-1alpha in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-gamma-angiotensin II receptor type 1 axis. Hypertension (Dallas, Tex. : 1979) 62:634–640
Kottgen M, Walz G (2005) Subcellular localization and trafficking of polycystins. Pflugers Arch 451:286–293
Krause SM (1999) Heterogeneous transmural gene expression of calcium-handling proteins and natriuretic peptides in the failing human heart. Cardiovasc Res 43:279–281
Kudej RK, Iwase M, Uechi M et al (1997) Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol 29:2735–2746
Kuo IY, Kwaczala AT, Nguyen L et al (2014) Decreased polycystin 2 expression alters calcium-contraction coupling and changes beta-adrenergic signaling pathways. Proc Natl Acad Sci U S A 111:16604–16609
Kuo IY, Duong SL, Nguyen L et al (2016) Decreased polycystin 2 levels result in non-renal cardiac dysfunction with aging. PLoS One 11:e0153632
Kurbegovic A, Cote O, Couillard M et al (2010) Pkd1 transgenic mice: adult model of polycystic kidney disease with extrarenal and renal phenotypes. Hum Mol Genet 19:1174–1189
Laine M, Id L, Vuolteenaho O et al (1996) Role of calcium in stretch-induced release and mRNA synthesis of natriuretic peptides in isolated rat atrium. Pflugers Arch 432:953–960
Lohse MJ, Engelhardt S, Eschenhagen T (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:896–906
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470–479
Luciano RL, Dahl NK (2014) Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant 29:247–254
Maltsev AV, Yaniv Y, Stern MD et al (2013) RyR-NCX-SERCA local cross-talk ensures pacemaker cell function at rest and during the fight-or-flight reflex. Circ Res 113:e94–e100
Martinez-Vea A, Valero FA, Bardaji A et al (2000) Left ventricular hypertrophy in hypertensive patients with autosomal dominant polycystic kidney disease: influence of blood pressure and humoral and neurohormonal factors. Am J Nephrol 20:193–200
Marx U, Lassmann G, Holzhutter HG et al (2000) Rapid flip-flop of phospholipids in endoplasmic reticulum membranes studied by a stopped-flow approach. Biophys J 78:2628–2640
Mcgrath MF, De Bold AJ (2005) Determinants of natriuretic peptide gene expression. Peptides 26:933–943
Morisco C, Zebrowski DC, Vatner DE et al (2001) Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol 33:561–573
Nauli SM, Alenghat FJ, Luo Y et al (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137
Nishikimi T, Maeda N, Matsuoka H (2006) The role of natriuretic peptides in cardioprotection. Cardiovasc Res 69:318–328
Orskov B, Sorensen VR, Feldt-Rasmussen B et al (2012) Changes in causes of death and risk of cancer in Danish patients with autosomal dominant polycystic kidney disease and end-stage renal disease. Nephrol Dial Transplant 27:1607–1613
Paavola J, Schliffke S, Rossetti S et al (2013) Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol 58:199–208
Patel A, Honore E (2010) Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol 6:530–538
Pedrozo Z, Criollo A, Battiprolu PK et al (2015) Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ channel protein stability. Circulation 131:2131–2142
Perrone RD, Abebe KZ, Schrier RW et al (2011) Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6:2508–2515
Puhl SL, Weeks KL, Ranieri A et al (2016) Assessing structural and functional responses of murine hearts to acute and sustained beta-adrenergic stimulation in vivo. J Pharmacol Toxicol Methods 79:60–71
Qian F, Germino FJ, Cai Y et al (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16:179–183
Saggar-Malik AK, Missouris CG, Gill JS et al (1994) Left ventricular mass in normotensive subjects with autosomal dominant polycystic kidney disease. BMJ 309:1617–1618
Schrier RW, Abebe KZ, Perrone RD et al (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371:2255–2266
Sergeeva IA, Christoffels VM (2013) Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim Biophys Acta 1832:2403–2413
Sharkey SW, Windenburg DC, Lesser JR et al (2010) Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 55:333–341
Todd GL, Baroldi G, Pieper GM et al (1985) Experimental catecholamine-induced myocardial necrosis. I. Morphology, quantification and regional distribution of acute contraction band lesions. J Mol Cell Cardiol 17:317–338
Todd GL, Baroldi G, Pieper GM et al (1985) Experimental catecholamine-induced myocardial necrosis. II. Temporal development of isoproterenol-induced contraction band lesions correlated with ECG, hemodynamic and biochemical changes. J Mol Cell Cardiol 17:647–656
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet (London, England) 369:1287–1301
Valero FA, Martinez-Vea A, Bardaji A et al (1999) Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 10:1020–1026
Vasan RS, Benjamin EJ, Larson MG et al (2002) Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA 288:1252–1259
Virzi GM, Corradi V, Panagiotou A et al (2010) ADPKD: prototype of cardiorenal syndrome type 4. Int J Nephrol 2011:490795
Wu G, Markowitz GS, Li L et al (2000) Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24:75–78
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516
Zhao Y, Haylor JL, Ong AC (2002) Polycystin-2 expression is increased following experimental ischaemic renal injury. Nephrol Dial Transplant 17:2138–2144
Acknowledgements
We thank Drs. Stefan Somlo and Yiqiang Cai (Yale University) for the PC2 antibody. We thank Lily Nguyen and Nicole Mikush for technical assistance. We acknowledge funding from the NIH to support the Yale Cell Biology Microscopy Core (5P30DK034989, OD020142). Grant support is acknowledged: a scholarship from the German National Merit Foundation (EG), a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brazil (FOL), K99DK101585 (IYK), 5P01DK057751, and P30DK090744 (BEE). Helpful discussions with Allison Brill are acknowledged.
Author information
Authors and Affiliations
Contributions
EG, IYK, and BEE conceived the project. EG, FOL, and YH conducted experiments and EG, YH, and FOL analyzed data. EG and FOL wrote the first draft of the manuscript, and all authors edited the manuscript. All authors agreed to the final manuscript.
Corresponding author
Ethics declarations
The Yale University Animal Ethics Committee (IACUC) approved the animal housing conditions and experimental procedures conducted in this study.
Competing interests
The authors state that there are no competing interests.
Electronic supplementary material
Supplemental Fig. 1
Expression of Polycystin-2 (PC2) is increased after prolonged β-adrenergic stress. Western blot analysis of PC2 expression in cardiac ventricular tissue of 6 week old male WT and Pkd2+/− mice after 7 days of i.p. injection of saline (control) or isoproterenol (ISO: 10 mg/kg/day; 25 mg/kg/day or 50 mg/kg/day). Representative image of the blots. Each lane represents a different animal. (PDF 263 kb)
Supplemental Fig. 2
Echocardiographic and hemodynamic analysis of 6 weeks old male WT (white bar) and Pkd2+/− (black bar) mice after 7 days of isoproterenol (ISO; 25 mg/kg/day) or vehicle treatment. (A) Systolic interventricular septum thickness; (B) Diastolic interventricular septum thickness; (C) Systolic interventricular septum thickness to left ventricular internal diameter ratio; (D) Diastolic interventricular septum thickness to left ventricular internal diameter ratio; (E) Systolic left ventricular internal diameter; (F) Diastolic left ventricular internal diameter; (G) Systolic left ventricular volume; (H) Diastolic left ventricular volume; (I) Ejection Fraction; (J) Fractional shortening. Results are presented as the mean ± sem. *means p < 0.05. Two-way ANOVA, Tukey post test. (PDF 78 kb)
Supplemental Fig. 3
WT and Pkd2+/− mice have comparable enhance of contraction force in dobutamine test. Hemodynamic analysis of rate of rise of left ventricular (LV) pressure during systole in 6 weeks old male WT and Pkd2+/− mice after 7 days of isoproterenol (ISO; 25 mg/kg/day) or vehicle treatment. Results are presented as the mean ± sem. **** means p < 0.001. Two-way ANOVA, Tukey post test. (PDF 33 kb)
Supplemental Table 1
Body weights after 3 days of rest following ISO treatment. Body weight (g) of WT and Pkd2+/− mice directly after 7 days of control (saline) or stress (isoproterenol (ISO; 25 mg/kg/day)) treatment (day 7) followed by 3 days of rest (day 10). (PDF 26 kb)
Rights and permissions
About this article
Cite this article
Giehl, E., Lemos, F.O., Huang, Y. et al. Polycystin 2-dependent cardio-protective mechanisms revealed by cardiac stress. Pflugers Arch - Eur J Physiol 469, 1507–1517 (2017). https://doi.org/10.1007/s00424-017-2042-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-2042-7