Abstract
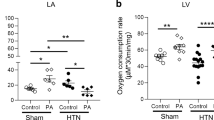
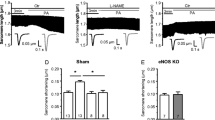
Cardiac neuronal nitric oxide synthase (nNOS) is an important molecule that regulates intracellular Ca2+ homeostasis and contractility of healthy and diseased hearts. Here, we examined the effects of nNOS on fatty acid (FA) regulation of left ventricular (LV) myocyte contraction in sham and angiotensin II (Ang II)-induced hypertensive (HTN) rats. Our results showed that palmitic acid (PA, 100 μM) increased the amplitudes of sarcomere shortening and intracellular ATP in sham but not in HTN despite oxygen consumption rate (OCR) was increased by PA in both groups. Carnitine palmitoyltransferase-1 inhibitor, etomoxir (ETO), reduced OCR and ATP with PA in sham and HTN but prevented PA potentiation of sarcomere shortening only in sham. PA increased nNOS-derived NO only in HTN. Inhibition of nNOS with S-methyl-l-thiocitrulline (SMTC) prevented PA-induced OCR and restored PA potentiation of myocyte contraction in HTN. Mechanistically, PA increased intracellular Ca2+ transient ([Ca2+]i) without changing Ca2+ influx via L-type Ca2+ channel (I-LTCC) and reduced myofilament Ca2+ sensitivity in sham. nNOS inhibition increased [Ca2+]i, I-LTCC and reduced myofilament Ca2+ sensitivity prior to PA supplementation; as such, normalized PA increment of [Ca2+]i. In HTN, PA reduced I-LTCC without affecting [Ca2+]i or myofilament Ca2+ sensitivity. However, PA increased I-LTCC, [Ca2+]i and reduced myofilament Ca2+ sensitivity following nNOS inhibition. Myocardial FA oxidation (18F-fluoro-6-thia-heptadecanoic acid, 18F-FTHA) was comparable between groups, but nNOS inhibition increased it only in HTN. Collectively, PA increases myocyte contraction through stimulating [Ca2+]i and mitochondrial activity in healthy hearts. PA-dependent cardiac inotropy was limited by nNOS in HTN, predominantly due to its modulatory effect on [Ca2+]i handling.







Similar content being viewed by others
References
Ashley CC, Moisescu DG (1977) Effect of changing the composition of the bathing solutions upon the isometric tension–pCa relationship in bundles of crustacean myofibrils. J Physiol 270(3):627–652
Best PM, Donaldson SK, Kerrick WG (1977) Tension in mechanically disrupted mammalian cardiac cells: effects of magnesium adenosine triphosphate. J Physiol 265(1):1–17
Briones AM, Touyz RM (2010) Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12(2):135–142
Briston SJ, Dibb KM, Solaro RJ, Eisner DA, Trafford AW (2014) Balanced changes in Ca buffering by SERCA and troponin contribute to Ca handling during beta-adrenergic stimulation in cardiac myocytes. Cardiovasc Res 104(2):347–354
Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cochemé HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP (2013) Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19(6):753–759
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113(6):709–724
Fabiato A, Fabiato F (1978) Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol 276:233–255
Fauconnier J, Andersson DC, Zhang SJ, Lanner JT, Wibom R, Katz A, Bruton JD, Westerblad H (2007) Effects of palmitate on Ca(2+) handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes 56(4):1136–1142
Godt RE (1974) Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol 63(6):722–739
Golowasch J, Thomas G, Taylor AL, Patel A, Pineda A, Khalil C, Nadim F (2009) Membrane capacitance measurements revisited: dependence of capacitance value on measurement method in nonisopotential neurons. J Neurophysiol 102(4):2161–2175
Hernandez AM, Huber JS, Murphy ST, Janabi M, Zeng GL, Brennan KM, O'Neil JP, Seo Y, Gullberg GT (2013) Longitudinal evaluation of left ventricular substrate metabolism, perfusion, and dysfunction in the spontaneously hypertensive rat model of hypertrophy using small-animal PET/CT imaging. J Nucl Med 54(11):1938–1945
Huke S, Knollmann BC (2010) Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol 48(5):824–833
Jin CZ, Jang JH, Kim HJ, Wang Y, Hwang IC, Sadayappan S, Park BM, Kim SH, Jin ZH, Seo EY, Kim KH, Kim YJ, Kim SJ, Zhang YH (2013) Myofilament Ca2+ desensitization mediates positive lusitropic effect of neuronal nitric oxide synthase in left ventricular myocytes from murine hypertensive heart. J Mol Cell Cardiol 60:107–115
Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM (2003) Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res 92(12):1322–1329
Kolwicz SC Jr, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113(5):603–616
Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ, Paterson DJ (2013) Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension 61(1):202–207
Liou YM, Kuo SC, Hsieh SR (2008) Differential effects of a green tea-derived polyphenol (−)-epigallocatechin-3-gallate on the acidosis-induced decrease in the Ca(2+) sensitivity of cardiac and skeletal muscle. Pflugers Arch 456(5):787–800
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1):207–258
Mancini A, Imperlini E, Nigro E, Montagnese C, Daniele A, Orrù S, Buono P (2015) Biological and nutritional properties of palm oil and palmitic acid: effects on health. Molecules 20(9):17339–17361
Massion PB, Feron O, Dessy C, Balligand JL (2003) Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93(5):388–398
Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA (2012) Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 59(22):1979–1987
Ohira H, deKemp R, Pena E, Davies RA, Stewart DJ, Chandy G, Contreras-Dominguez V, Dennie C, Mc Ardle B, Mc Klein R, Renaud JM, DaSilva JN, Pugliese C, Dunne R, Beanlands R, Mielniczuk LM (2015) Shifts in myocardial fatty acid and glucose metabolism in pulmonary arterial hypertension: a potential mechanism for a maladaptive right ventricular response. Eur Heart J Cardiovasc Imaging 17(12):1424–1431
Pellieux C, Montessuit C, Papageorgiou I, Lerch R (2009) Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-alpha. Cardiovasc Res 82(2):341–350
Recchia FA, McConnell PI, Loke KE, Xu X, Ochoa M, Hintze TH (1999) Nitric oxide controls cardiac substrate utilization in the conscious dog. Cardiovasc Res 44(2):325–332
Robinson P, Griffiths PJ, Watkins H, Redwood CS (2007) Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 101(12):1266–1273
Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res 92(5):e52–e59
Shah AM, MacCarthy PA (2000) Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther 86(1):49–86
Takahashi R, Shimazaki Y, Endoh M (2001) Decrease in Ca(2+)-sensitizing effect of UD-CG 212 Cl, a metabolite of pimobendan, under acidotic condition in canine ventricular myocardium. J Pharmacol Exp Ther 298(3):1060–1066
Torres J, Darley-Usmar V, Wilson MT (1995) Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem J 312(Pt 1):169–173
Welter R, Yu L, Yu CA (1996) The effects of nitric oxide on elecontrolon transport complexes. Arch Biochem Biophys 331(1):9–14
Zhang YH (2016) Neuronal nitric oxide synthase in hypertension—an update. Clin Hypertens 22:20
Zhang YH, Casadei B (2012) Sub-cellular targeting of constitutive NOS in health and disease. J Mol Cell Cardiol 52(2):341–350
Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B (2008) Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 102(2):242–249
Zhang YH, Dingle L, Hall R, Casadei B (2009) The role of nitric oxide and reactive oxygen species in the positive inotropic response to mechanical stretch in the mammalian myocardium. Biochim Biophys Acta 1787(7):811–817
Zhang YH, Jin CZ, Jang JH, Wang Y (2014) Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. J Physiol 592(15):3189–3200
Zhao ZH, Jin CL, Jang JH, Wu YN, Kim SJ, Jin HH, Cui L, Zhang YH (2016) Assessment of myofilament Ca2+ sensitivity underlying cardiac excitation–contraction coupling. J Vis Exp (114)
Zhao ZH, Youm JB, Wang Y, Lee JH, Sung JH, Kim JC, Woo SH, Leem CH, Kim SJ, Cui L, Zhang YH (2016) Cardiac inotropy, lusitropy, and Ca2+ handling with major metabolic substrates in rat heart. Pflugers Archiv 468(11–12):1995–2006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study protocol was in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and also conforms to the Institutional Animal Care and Use Committee (IACUC) in Seoul National University (IACUC approval no.: SNU-101213-1; SNU-160119-4-1).
Conflicts of interests
None.
Sources of funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013068067), the Brain Korea 21 Graduate Programme of the Korean Ministry of Education, Science and Technology, Seoul National University Hospital, the Korean Society of Hypertension (2013), SK Telecom Research Fund (no. 3420130290), and the National Natural Science Foundation of China (NSFC, 31460265).
Electronic supplementary material
ESM 1
Supplementary Fig. 1. Effects of ROS scavenger, tiron, on PA regulation of myocyte contraction in sham and in HTN rats. a–c Representative raw traces and mean values of sarcomere shortening by PA with tiron (1 mM). Sarcomere shortening was remained increased by PA in sham rats and was unaffected by PA in HTN rats, despite tiron pre-treatment. Supplementary Fig. 2. Examination of FA uptake with PET/CT in sham and HTN rats. a. PET/CT images were obtained by using 18F-FTHA in the LV at basal, in the presence of nNOS inhibitor, SMTC (1 mg/kg) and l-NAME (5 mg/kg). b. Averaged mean values of 18F-FTHA uptake in the myocardium/liver between sham and HTN with and without NOS inhibition. nNOS inhibition induced small but significant increase in 18F-FTHA uptake in HTN rats only. l-NAME significantly increased 18F-FTHA uptake in both groups, with an effect significantly higher in sham rats compared to that in HTN rats. Supplementary Fig. 3. PA regulation of the peak ICa-L density and inactivation kinetics with and without nNOS inhibition in sham and HTN rats. LTCC was stimulated by a step depolarization protocol from −60 to +40 mV (10 mV interval) for 200 ms (holding potential, −40 mV). a–d Representative ICa-L density with and without PA before and after nNOS inhibition (SMTC, 100 nM, 30 min–1 h). PA slightly but significantly reduced peak ICa-L density at 0 mV in both groups. nNOS inhibition increased ICa-L density at 0 mV and abolished PA regulation in both groups. e–h Inactivation of ICa-L was slowed by PA in both groups; nNOS inhibition (SMTC, 100 nM, 30 min–1 h) significantly reduced inactivation and abolished PA-dependent responses in sham and in HTN rats. Supplementary Fig. 4. PA regulation of the Ca2+ influx through LTCC. LTCC was stimulated by a step depolarization protocol from −60 to +40 mV (10 mV interval) for 200 ms (holding potential, −40 mV). a & c. Representative ICa-L integral at 0 mV in sham and HTN rats before and after nNOS inhibition with and without PA. b & d. Averaged mean values showed that PA did not change ICa-L integral in sham rats but significantly reduced it in HTN rats. nNOS inhibition increased ICa-L integral with and without PA in both groups and abolished the response of PA in two groups. (PPTX 2.74 MB)
Rights and permissions
About this article
Cite this article
Jin, C.L., Yin, M.Z., Paeng, J.C. et al. Neuronal nitric oxide synthase modulation of intracellular Ca2+ handling overrides fatty acid potentiation of cardiac inotropy in hypertensive rats. Pflugers Arch - Eur J Physiol 469, 1359–1371 (2017). https://doi.org/10.1007/s00424-017-1991-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1991-1