Abstract
Purpose
This pilot study investigated differences in lean tissue mass, muscle strength, muscle quality (strength per unit of muscle mass; MQ), and functional performance in healthy younger and older individuals. The most robust predictors of appendicular lean mass (ALM) were then determined in each group.
Methods
Fifty younger (18–45 years) and 50 older (60–80 years) participants completed tests of upper and lower body strength alongside body composition by dual-energy X-ray absorptiometry from which upper- and lower-body MQ were estimated. Available cut-points for older people were used to determine low upper-body MQ in both groups. Low lower-body MQ was determined as at least two standard deviations below the mean of the younger group. Functional performance was assessed by gait speed. Sarcopenia was identified using two established definitions.
Results
Upper and lower body strength, ALM, lower-body MQ and gait speed were significantly higher in the younger group (all p < 0.002). Sarcopenia was identified in 2–4% of the older group. Low upper-body MQ was evident in 32% and 42% of the younger and older group, respectively. Low lower-body MQ was observed in 4% of younger participants, and 50% of older participants. In both groups, the most robust predictors of ALM were upper and lower body strength (young R2 = 0.74, 0.82; older R2 = 0.68, 0.72).
Conclusions
Low MQ despite low prevalence rates of sarcopenia in both groups suggests a need for age-specific MQ cut-points. Muscle quality assessments might be useful complementary prognostic tools alongside existing sarcopenia definitions.
Similar content being viewed by others
Introduction
The progressive loss of skeletal muscle mass and strength with age is associated with a number of adverse health outcomes, such as decreased quality of life, functional impairment, disability, increased risk of falls, hospitalisation, and mortality (Batsis et al. 2015; Beaudart et al. 2017; Lauretani et al. 2003; Bischoff-Ferrari et al. 2015; Cesari et al. 2009; Gariballa and Alessa 2013; Guralnik et al. 2000; Landi et al. 2013). The early identification of individuals with low muscle mass and impaired physical function may promote desirable patient outcomes over the long-term. The term sarcopenia was first used to describe the age-associated decline in skeletal muscle mass (Rosenberg 1989). This definition has expanded to include measures of both skeletal muscle mass and function to determine the presence of sarcopenia (Cruz-Jentoft et al. 2010; Goodpaster et al. 2006). The absence of a consensus definition for sarcopenia represents a considerable challenge for assessing its prevalence and impact on public health (Beaudart et al. 2014; Mayhew et al. 2018; Batsis et al. 2015). Importantly, the prevalence of sarcopenia appears to be greater when assessing muscle mass only (24.2–40.4%) as opposed to measures of muscle mass, strength and/or physical function in tandem (9.9–18.6%) (Mayhew et al. 2018).
Muscle quality (MQ), typically defined as muscle strength or power per unit of muscle mass (Barbat-Artigas et al. 2012), is a key determinant of muscle function in later life (McGregor et al. 2014), and declines with age (Newman et al. 2003). A number of factors may mediate this, such as a decrease in the number and cross-sectional area of type II fibres, fat infiltration, and neurological derangements (McGregor et al. 2014; Fragala et al. 2015). Therefore, the inclusion of MQ assessment to augment existing sarcopenia definitions might serve as a more effective means of identifying individuals at risk of impaired mobility in later life (Cruz-Jentoft et al. 2018). Paradoxically, cut-points for handgrip strength have been lowered by the European Working Group on Sarcopenia in Older People (EWGSOP) (Cruz-Jentoft et al. 2018) in comparison with previous recommendations (Cruz-Jentoft et al. 2010). This reduces emphasis on MQ, since strength is a component of MQ determination (Barbat-Artigas et al. 2012).
In addition to assessing upper body strength, the assessment of lower body strength and function in older adults is important, since age-related declines are faster in lower body than upper body strength (Goodpaster et al. 2006; Janssen et al. 2000). Incorporation of upper and lower body strength is preferential to upper body strength alone (Yeung et al. 2018). In addition, low knee extension strength is a better predictor of mortality risk than low handgrip strength (Mitchell et al. 2012; Laukkanen et al. 1995), and handgrip strength alone does not provide a good measure to evaluate the effectiveness of interventions in frail populations (Tieland et al. 2015).
Methods employed to assess lower body strength are varied, whereby isokinetic dynamometry (Krause et al. 2012; Newman et al. 2006; Bouchard et al. 2011), a force transducer (Yeung et al. 2018), spring gauge (Hairi et al. 2010), and adopted proxy measures (Cruz-Jentoft et al. 2018) have been used. Isokinetic dynamometry is often considered the ‘gold standard’ for lower-body strength testing and has been used consistently in studies with older populations (Lauretani et al. 2003; Brach et al. 2004; Newman et al. 2003, 2006; Goodpaster et al. 2006; Scott et al. 2014). The availability of such costly and large-scale equipment to assess lower body strength might be restricted in clinical and epidemiological settings (Martin et al. 2006; Yeung et al. 2018). Therefore, the use of practical exercise training-specific equipment to establish leg extension strength might be a more cost-effective and accessible alternative to isokinetic dynamometry. For instance, leg extension one-repetition maximum (1RM) strength is strongly associated with peak torque values obtained using isokinetic dynamometry (r = 0.78–0.88), and to a greater extent than a leg press 1RM test (r = 0.72–0.77) (Verdijk et al. 2009). The need for lower-body strength testing as part of the comprehensive geriatric assessment was highlighted by Yeung et al. (2018), who demonstrated stronger associations of health characteristics with knee extension strength in comparison with handgrip strength in community-dwelling older adults. All of the above emphasise the need to assess lower body strength and muscle quality in older populations as a means to identify individuals at risk of impaired physical function and/or sarcopenia.
The objectives of this pilot study were to investigate differences in lean tissue mass, muscle strength, functional performance, and physical function among healthy young and older individuals and establish the relationships of these variables with total appendicular lean mass (ALM). We then sought to assess the presence of sarcopenia using two established definitions with the lowest and highest pooled prevalence estimates (Mayhew et al. 2018). We also performed MQ assessment to identify individuals with evidence of functional decline and aimed to reconcile these findings with sarcopenia definitions.
Materials and methods
Study design
A cross-sectional study involving two testing sessions, separated by a minimum period of 7 days but no longer than 14 days, was conducted between October 2016 and July 2017. In the first visit, measures of strength, body composition, functional performance and physical activity were taken and participants were familiarised with the lower body 1RM test. On the second visit, participants completed the 1RM test only. All procedures were conducted by the same trained researcher at the same time of day, under strict controls to mitigate the impact of confounding variables such as exercise and diet.
Participants
Fifty younger (18–45 years; 30 males, 20 females) and 50 older (60–80 years; 24 males, 26 females) participants were randomly recruited from local universities and the wider community, using a combination of email communications, online or printed advertisements and leaflets. Participant characteristics for both groups are provided in Table 1. All participants were independently living and free from cardiovascular, metabolic, endocrine, or other metabolic disease. Participants were excluded if they smoked, used oestrogens within the previous 3 months, had a pacemaker, or were taking any medication known to affect protein metabolism (i.e., anabolic steroids, corticosteroids, and peripheral vasodilators). Younger females were excluded if they were using the oral contraceptive pill. Participants were randomly recruited from a range of sports teams and activity groups, as well as from the lay public, to represent a broad range of physical activity levels. Written informed consent was provided prior to commencing the study and all procedures were carried out in accordance with the Helsinki Declaration of 1964, following approval by the University Faculty Research Ethics Committee.
Preliminary screening and basic anthropometry
Participants arrived at the laboratory between 07.00 and 09.00 in a fasted, euhydrated state after avoiding alcohol and exercise for a 24 h period. Following an initial briefing, resting heart rate (RHR), systolic and diastolic blood pressure (SBP and DBP, respectively) were measured using a manual sphygmomanometer (Accoson Greenlight 300, Accoson, United Kingdom). Stature (to the nearest 0.1 cm) and body mass (to the nearest 0.1 kg) were recorded using a stadiometer (Seca 220, Hamburg, Germany) and calibrated electronic scales (Seca 220, Hamburg, Germany). Body mass index (BMI) was derived by calculating mass/height2. Finger capillary blood samples were obtained and analysed for total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides and glucose using a Cholestech LDX (Alere San Diego, Inc., San Diego, CA) capillary whole blood lipid analyser (Donato et al. 2015).
Body composition assessment
Total-body fat mass, lean tissue mass, bone mineral content and percentage tissue fat mass (%TFM) values were ascertained by one total-body dual-energy X-ray absorptiometry (DXA) scan (GE Lunar iDXA, GE Healthcare, Madison, WI). These data were used to calculate appendicular lean mass (ALM). Participants were scanned in a fasted, euhydrated state as per established recommendations (Nana et al. 2012; Sawka et al. 2007). Participants removed shoes and jewellery before receiving the scan, whilst adopting a supine position with arms to the side in the semi-prone position (Thurlow et al. 2018) and ankles supported with the Lunar ankle strap (0.5 cm space between the ankles). Standard mode scans took approximately 7.5 min to complete, whereas those > 100 kg in body mass (n = 2) necessitated the use of the thick mode scan, which took approximately 12.5 min.
The values for the body composition outcomes were determined from the ratio of soft tissue attenuation of two X-ray energy beams for each pixel containing a minimal amount of soft tissue but no significant bone (Mazess et al. 1990). In our laboratory, the in vivo short-term precision (%CV) for total-body composition variables are 0.82% for fat mass, 0.51% for lean mass, 0.86% for percentage body fat and 0.60% for bone mineral content (Hind et al. 2011). The machine was checked and calibrated on a daily basis in line with the manufacturer’s recommendations. All scanning and analysis procedures were performed by the same trained operator using the Lunar enCORE software package (version 15.0).
Functional performance and strength testing
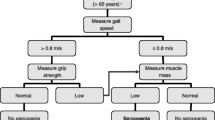
Gait speed was measured directly using a 6-m course and photoelectric timing gates (Witty, Microgate, Bolzano, Italy). Participants were instructed to walk at their usual pace between two gates, with the fastest of these attempts used for the final analysis. The established cut-point of < 1 m/s was used to characterise impaired physical performance (Cesari et al. 2005, 2009; Visser et al. 2002).
Dominant and non-dominant handgrip strength (specific force, kg) were assessed using a Takei T.K.K. 5401 GRIP-D handgrip dynamometer (Takei Scientific Instruments Co., Ltd, Niigata, Japan), with the participant in a seated position (knees at 90°) and the shoulders adducted and neutrally rotated. The elbow was flexed at 90° with the forearm in a neutral position and the wrist in slight extension (0°–30°) (De Dobbeleer et al. 2017). The participant was then asked to squeeze the handle of the dynamometer as hard as possible for a maximum of five seconds. The highest of three attempts was noted and the process was repeated for the non-dominant hand. The previously established cut-points of < 30 kg for males and < 20 kg for females, respectively, were used for the determination of low grip strength (Lauretani et al. 2003).
For testing of lower body strength, participants were asked to perform separate 1RM tests of the dominant and non-dominant limbs using a Cybex VRS Prestige (Cybex International, Medway, MA) leg extension machine. A 5 min dynamic warmup was performed, with emphasis on the lower body musculature, prior to beginning a protocol-specific warm-up. Each participant’s 1RM was measured using previously described procedures (Baechle and Earle 2008). At the initial laboratory visit, participants completed a 1RM test for familiarisation purposes which was then repeated on the subsequent visit to establish 1RM.
Assessment of habitual physical activity
Participants were asked to provide estimates of physical activity over the previous 7 days, using the long form of the International Physical Activity Questionnaire (IPAQ). The estimated total time spent in the respective domains of occupational, transport, household and leisure-related physical activity were calculated. These data were then used to determine weighted MET-minutes per week (MET min/week) for walking, moderate and vigorous activity, respectively (Craig et al. 2003). For descriptive purposes, participants were classified as high, moderate, or low according to IPAQ categorical criteria. Highly active was defined as 7 or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities, achieving a minimum total physical activity of ≥ 3000 MET-min/week. Moderately active was defined as 5 or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities, amounting to a minimum total physical activity of ≥ 600 MET-min/week. Participants were classified as low if they did not meet the criteria for the moderate category.
Sarcopenia classification and muscle quality
Two established definitions were applied to the sample groups to establish the prevalence of sarcopenia. ALM was calculated as the sum of the fat-free mass in the limbs, excluding bone mineral content, divided by body mass and expressed as a percentage. This definition has been applied and revealed a 40.4% prevalence rate of sarcopenia (Mayhew et al. 2018). In the present study, cut-points of < 27.1% for males and < 22.3% for females were used to define sarcopenia.
The second definition was the composite measure of the International Working Group on Sarcopenia (IWGS) (Fielding et al. 2011), representing the lowest prevalence limit (9.9%) according to Mayhew et al. (2018). Participants with a gait speed of < 1 m/s were identified and subsequently screened by lean tissue mass. ALM/height2 was calculated and the cut-points of ≤ 7.23 kg/m2 for males and ≤ 5.67 kg/m2 for females were applied to determine sarcopenia.
Upper-body MQ was calculated by dividing the maximum handgrip strength by upper body ALM, with cut-points determined as < 5.76 kg/kg for males and < 5.475 kg/kg for females, respectively (Cooper et al. 2014). Lower-body MQ was determined as the ratio of dominant leg extension strength to leg lean mass (Hairi et al. 2010). Since to our knowledge cut-points for younger populations do not currently exist, cautious and limited comparisons of upper-body MQ for the younger group were made against established norms for the older group. Protocol-specific cut-points for lower-body MQ using the adopted MQ algorithm do not currently exist. Therefore, and in line with the selection of cut-points for sarcopenia (Baumgartner et al. 1998), low MQ in the lower body was classified as at least two standard deviations below the mean of the corresponding younger group in the present study, with cut-points of ≤ 2.11 kg/kg for males and ≤ 1.56 kg/kg for females, respectively.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics software (Version 24.0, IBM Corp., Armonk, NY). Prior to analysis, assumptions of normality in the data were made using Shapiro–Wilk tests and visualisation of normality plots. Between-group differences were investigated using multivariate analysis of variance (MANOVA). Cohen’s d effect sizes were calculated to quantify the magnitude of differences in body composition, functional and strength testing, MQ, and estimates of physical activity. The effect size thresholds were classified in line with Cohen (1988): < 0.2 = trivial; 0.2–0.5 = small; 0.5–0.8 = medium; 0.8–1.3 = large; > 1.3 = very large.
The relationships between total ALM and strength, functional performance and physical activity were determined for each group using Pearson’s correlation coefficients. The strength of the relationship was classified according to the thresholds of Cohen (1988): small = 0.10, moderate = 0.30, large = 0.50. Fisher r-to-z transformations were performed to assess the significance of differences in correlation coefficients between groups.
By way of multiple regression analysis using the forced entry method, the best predictors of upper and lower body ALM were determined for both sample groups. Adjusted R2 and multicollinearity statistics (tolerance and variance inflation factor) were used to identify the optimum equations (Krause et al. 2012; Stoever et al. 2017). For all regression models, dominant handgrip strength and dominant leg extension strength were chosen as the best predictors. For all appropriate analyses, 95% confidence intervals (95% CI) are reported. All data are presented as mean ± standard deviation (SD) with statistical significance for all analyses set at P ≤ 0.05.
Results
Basic anthropometry and preliminary screening
Comparisons of basic anthropometry and preliminary screening measures are given in Table 1. The younger group was significantly taller and had lower resting systolic blood pressure than the older group. No significant differences were found for body mass, BMI, resting heart rate, or resting diastolic blood pressure. Blood lipid profile components and blood pressure in both groups were within established norms for metabolically healthy individuals (data not shown).
Body composition assessment
Comparisons of body composition, functional measures and strength testing are presented in Table 2. The younger group had significantly lower fat mass and %TFM than the older group. Lean mass and total ALM were both significantly higher in the younger group.
In both groups, lean mass was strongly and significantly associated with total ALM (Table 3). In the older group only, there was a moderate positive correlation between total ALM and fat mass. In the younger group, fat mass was negatively associated with lean mass (r = -0.22), however this was not statistically significant (P = 0.130). In the older group, fat mass was positively associated with lean mass (r = 0.53; P < 0.0001). All correlation coefficients were significantly different between groups.
Functional measures and strength testing
Upper and lower-body strength testing revealed significantly greater strength in the younger group (Table 2). No significant differences were found in upper-body MQ, whereas lower-body MQ was significantly greater in the younger group. Gait speed was significantly faster in the younger group.
In both groups, large and significant positive associations were found between upper and lower body strength and total ALM (Table 3). Negative associations were found between upper-body MQ and total ALM in both groups. In addition, a large and significant positive association was found between lower-body MQ and total ALM in the younger group. None of the coefficients differed significantly between groups except for dominant leg extension strength and lower-body MQ.
Assessment of habitual physical activity
The younger group demonstrated significantly greater levels of vigorous activity than the older group. All other comparisons were not significantly different between groups. No significant associations were found between total ALM and IPAQ-derived data in either group, and the correlation coefficients did not differ significantly between groups.
Using the IPAQ categorical classification criteria, the younger group was profiled as follows: low = 2, moderate = 7, high = 41. The older group was as follows: low = 1, moderate = 16, high = 33.
Sarcopenia classification and muscle quality status
As per the definitions put forward by the IWGS, one older male was classified as sarcopenic. When sarcopenia was calculated using lean tissue mass as the sole measure, derived from ALM/body mass, only two older males were classified as sarcopenic.
Upper-body MQ was below established cut-points in 16 young (14 males, 2 females) and 21 older (14 males, 7 females) participants. For lower-body MQ, 17 older males and 8 older females were at least 2 standard deviations below the mean of the corresponding younger group. Nine older males had low MQ at both the upper and lower body, whereas this was only the case in two older females.
Multiple regression analyses
The results of the multiple regression models for the younger group are shown in Table 4. These models accounted for 82% and 74% of the variance in upper and lower body ALM, respectively, using dominant handgrip strength and dominant leg extension strength as predictor variables. The complete equations are as follows:
The same multiple regression models were applied to the older group and are shown in Table 5. The models accounted for 72% and 68% of the variance in upper and lower body ALM, using the same respective predictors. The complete equations are as follows:
Discussion
The aims of the present pilot study were to investigate differences in lean tissue mass, functional capacity and physical activity between younger and older individuals and to establish relationships between these variables and total ALM. Muscle quality assessment was performed at the upper and lower body, and these findings were reconciled with two established definitions of sarcopenia. Finally, regression analyses were performed to investigate the application of upper and lower body ALM for use as a proxy measure of MQ. The major findings of this study suggest that in spite of low sarcopenia rates (2–4% using two established definitions) in our older population, 42% had low upper-body MQ, as per existing thresholds. Interestingly, 32% of the younger group were also below these thresholds. In spite of the absence of protocol-specific benchmarks for lower-body MQ, we found that 50% of the older group had MQ values at least two standard deviations below the mean for the respective younger groups.
Our findings show that the younger group possessed lower fat mass and %TFM than the older group. Greater lean mass and ALM were also found in the younger group, with all comparisons supported by large effect sizes. These observations corroborate those of previous studies, suggesting that fat mass increases and lean mass decreases with age, without substantial differences in body mass (St-Onge and Gallagher 2010; Mazariegos et al. 1994; Atlantis et al. 2008).
Fat mass was positively correlated with both total ALM and lean mass in the older group only, and these findings concur with previous studies (Bouchard et al. 2011; Lebrun et al. 2006). This relationship may be important for clinical outcomes. Low fat mass and lean tissue mass pose the greatest risk of all-cause mortality in older individuals (Spahillari et al. 2016). Conversely, greater lean tissue mass is associated with improved overall mortality in older individuals (Spahillari et al. 2016). Greater fat mass is associated with a decreased risk of adverse events in hospitalised elderly patients (Bouillanne et al. 2009). Additionally, the overweight classification for the older group in the present study is associated with the lowest mortality rate in this population (Chang et al. 2012; Kvamme et al. 2012; Bouillanne et al. 2009). Together, these findings support the notion that lean tissue mass improvements should be emphasised in older adults ahead of measures such as BMI (Spahillari et al. 2016).
In the present study, maximal strength at the upper and lower body was significantly greater in the young, constituting large and very large effects, respectively. These findings are in line with previous literature that showed greater torque, normalised torque and power in younger versus older men, with the lower body affected more substantially than the upper body (Candow and Chilibeck 2005). Gait speed was significantly lower in the older group (moderate effect), and our findings correspond with Laufer (2005), who observed reduced forward walking velocity in older compared to younger individuals.
Upper-body MQ did not differ significantly between groups (small effect). In the older group, upper-body MQ was similar to that observed by Hairi et al. (2010), albeit in the 80–84 year old age group. Low upper-body MQ was prevalent in 42% of the older group, and in 32% of the younger group based on established cut-points (Cooper et al. 2014). The latter finding should be treated with caution since the aforementioned cut-points are age-specific. Nevertheless, this emphasises the need for further work to develop and establish age-specific MQ cut-points. The negative associations between total ALM and upper-body MQ in both groups are explained as a function of the MQ equation, in that the denominator (total ALM) includes tissue (i.e., lower body ALM) that has no impact on force production during handgrip strength assessment.
Lower-body MQ was significantly greater in the young (very large effect). It must also be noted that no protocol-specific cut-point thresholds have been defined for lower-body MQ metrics in young and older individuals, and this represents an avenue for future research. In contrast with upper-body MQ, lower-body MQ was positively associated with total ALM in the young; however, no effect was found for this variable in the older group. The absence of an association between total ALM and lower-body MQ in the older group warrants further exploration. Muscle quality has been shown to decline progressively with age, potentially as a result of neurological factors, such as decrements in excitation contraction coupling and motor nerve conduction velocity (Moore et al. 2014; Clark and Manini 2012). Therefore, our findings suggest that measures of lean mass and sarcopenia definitions cannot solely be used to determine musculoskeletal health in young and older adults, and reinforce the rationale for MQ assessment of the upper and lower body (Cruz-Jentoft et al. 2018).
Self-reported physical activity was significantly higher in the younger group for vigorous activities (moderate effect), whereas all other comparisons were non-significant between groups. No significant associations were found between total ALM and IPAQ-derived data in each group and the correlation coefficients did not significantly differ. These findings lend further support to the use of strength testing to predict ALM, whilst demonstrating that IPAQ data cannot be used to predict ALM.
In totality, our findings lend support for the assessment of lower-body strength testing and reinforce the importance of this metric as a key predictor of physical performance and function (Marsh et al. 2006; Coelho-Junior et al. 2018; Yeung et al. 2018; Bouchard et al. 2011). The use of handgrip strength testing is ubiquitous in the literature (Bischoff-Ferrari et al. 2015; Newman et al. 2003, 2006; Stoever et al. 2017; Lauretani et al. 2003), given its use as a highly efficient screening tool (Martin et al. 2006). Despite this, handgrip strength may misclassify individuals as it only accounts for ~ 40% of the variance in lower body strength (Manini and Clark 2012). Moreover, caution should be observed when characterising overall strength based on the use of a single measurement tool (Bohannon 2008; Mitchell et al. 2012; Tieland et al. 2015). In older adults, differential associations have been noted between health characteristics, knee extension strength and handgrip strength, to the extent that lower-body strength testing poses considerable additive value (Yeung et al. 2018). Therefore, we support the inclusion of lower-body strength testing in addition to that of handgrip strength to enable better prediction of ALM in older adults. The measurement of leg extension strength using cost-effective and practical equipment appears to be a useful alternative to isokinetic dynamometry, and aligns well with recent recommendations concerning strength testing in older adults (Cruz-Jentoft et al. 2018).
In conclusion, an important finding of the present study was the low number of older individuals identified as sarcopenic, and the similarity of two established definitions in detecting these individuals despite large pooled prevalence differences between definitions (Mayhew et al. 2018). Deficits in upper-body MQ were found in the younger (n = 16) and older (n = 21) groups. Furthermore, 25 older individuals had low MQ at the lower body. Eleven of the older individuals had low ‘global’ MQ (i.e., upper and lower), representing 22% of the older group. These findings lend support to the rationale for conducting MQ testing alongside established sarcopenia definitions to effectively identify individuals at risk of functional decline. Future studies should seek to investigate the veracity of our regression models in a larger and more diverse sample population. In this manner, MQ at the upper and lower body might be estimated using strength measures and implemented as a practical prognostic tool. Individuals with low ‘estimated’ MQ may then be referred for DXA imaging as necessary to confirm or deny the presence of sarcopenia and/or impaired MQ.
Abbreviations
- %TFM:
-
Percentage tissue fat mass
- 1RM:
-
One-repetition maximum
- ALM:
-
Appendicular lean mass
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- DXA:
-
Dual-energy X-ray absorptiometry
- HGS:
-
Handgrip strength
- IPAQ:
-
International Physical Activity Questionnaire
- IWGS:
-
International Working Group on Sarcopenia
- MANOVA:
-
Multivariate analysis of variance
- MET:
-
Metabolic equivalent
- MQ:
-
Muscle quality
- RHR:
-
Resting heart rate
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- VIF:
-
Variance inflation factor
References
Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA (2008) Lifestyle factors associated with age-related differences in body composition: the Florey Adelaide Male Aging Study. Am J Clin Nutr 88(1):95–104. https://doi.org/10.1093/ajcn/88.1.95
Baechle TR, Earle RW (2008) Essentials of strength training and conditioning, 3rd edn. Human Kinetics, Champaign
Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M (2012) How to assess functional status: a new muscle quality index. J Nutr Health Aging 16(1):67–77. https://doi.org/10.1007/s12603-012-0004-5
Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ (2015) Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res 35(12):1031–1039. https://doi.org/10.1016/j.nutres.2015.09.003
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross R, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Beaudart C, Rizzoli R, Bruyère O, Reginster J, Biver E (2014) Sarcopenia: burden and challenges for public health. Arch Public Health. https://doi.org/10.1186/2049-3258-72-45
Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O (2017) Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 12(1):e0169548. https://doi.org/10.1371/journal.pone.0169548
Bischoff-Ferrari HA, Orav JE, Kanis JA, Rizzoli R, Schlogl M, Staehelin HB, Willett WC, Dawson-Hughes B (2015) Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos Int 26 (12):2793–2802. https://doi.org/10.1007/s00198-015-3194-y
Bohannon RW (2008) Is it legitimate to characterize muscle strength using a limited number of measures? J Strength Cond Res Natl Strength Cond Assoc 22(1):166–173. https://doi.org/10.1519/JSC.0b013e31815f993d
Bouchard DR, Héroux M, Janssen I (2011) Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J Aging Health 23(2):313–328. https://doi.org/10.1177/0898264310388562
Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, Aussel C (2009) Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr 90(3):505–510. https://doi.org/10.3945/ajcn.2009.27819
Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB (2004) The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc 52(4):502–509
Candow DG, Chilibeck PD (2005) Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci 60(2):148–156
Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M (2005) Prognostic value of usual gait speed in well-functioning older people—results from the health, aging and body composition study. J Am Geriatr Soc 53(10):1675–1680. https://doi.org/10.1111/j.1532-5415.2005.53501.x
Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, Brach JS, Tylavsky FA, Satterfield S, Bauer DC, Rubin SM, Visser M, Pahor M (2009) Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc 57(2):251–259. https://doi.org/10.1111/j.1532-5415.2008.02126.x
Chang S-H, Beason TS, Hunleth JM, Colditz GA (2012) A systematic review of body fat distribution and mortality in older people. Maturitas 72(3):175–191. https://doi.org/10.1016/j.maturitas.2012.04.004
Clark BC, Manini TM (2012) What is dynapenia? Nutrition 28(5):495–503. https://doi.org/10.1016/j.nut.2011.12.002
Coelho-Junior HJ, Rodrigues B, Goncalves IO, Asano RY, Uchida MC, Marzetti E (2018) The physical capabilities underlying timed “up and go” test are time-dependent in community-dwelling older women. Exp Gerontol 104:138–146. https://doi.org/10.1016/j.exger.2018.01.025
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. Lawrence Erlbaum, Hillsdale
Cooper R, Hardy R, Bann D, Aihie Sayer A, Ward KA, Adams JE, Kuh D (2014) Body mass index from age 15 years onwards and muscle mass, strength, and quality in early old age: findings from the MRC national survey of health and development. J Gerontol Ser A Biol Sci Med Sci 69(10):1253–1259. https://doi.org/10.1093/gerona/glu039
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35(8):1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older P, the Extended Group for E (2018) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. https://doi.org/10.1093/ageing/afy169
De Dobbeleer L, Beyer I, Njemini R, Pleck S, Zonnekein N, Mets T, Bautmans I (2017) Force-time characteristics during sustained maximal handgrip effort according to age and clinical condition. Exp Gerontol 98(Supplement C):192–198. https://doi.org/10.1016/j.exger.2017.08.033
Donato LJ, Deobald GR, Wockenfus AM, Hornseth JM, Saenger AK, Karon BS (2015) Comparison of two point of care devices for capillary lipid screening in fasting and postprandial adults. Clin Biochem 48(3):174–176. https://doi.org/10.1016/j.clinbiochem.2014.11.003
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 12(4):249–256. https://doi.org/10.1016/j.jamda.2011.01.003
Fragala MS, Kenny AM, Kuchel GA (2015) Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med 45(5):641–658. https://doi.org/10.1007/s40279-015-0305-z
Gariballa S, Alessa A (2013) Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 32(5):772–776. https://doi.org/10.1016/j.clnu.2013.01.010
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB (2000) Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55(4):M221–M231
Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ, Sambrook PN (2010) Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc 58(11):2055–2062. https://doi.org/10.1111/j.1532-5415.2010.03145.x
Hind K, Oldroyd B, Truscott JG (2011) In vivo precision of the GE Lunar iDXA densitometer for the measurement of total body composition and fat distribution in adults. Eur J Clin Nutr 65(1):140–142. https://doi.org/10.1038/ejcn.2010.190
Janssen I, Heymsfield SB, Wang Z, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 year. J Appl Physiol 89:81–88
Krause KE, McIntosh EI, Vallis LA (2012) Sarcopenia and predictors of the fat free mass index in community-dwelling and assisted-living older men and women. Gait Posture 35(2):180–185. https://doi.org/10.1016/j.gaitpost.2011.09.003
Kvamme JM, Holmen J, Wilsgaard T, Florholmen J, Midthjell K, Jacobsen BK (2012) Body mass index and mortality in elderly men and women: the Tromso and HUNT studies. J Epidemiol Community Health 66(7):611–617. https://doi.org/10.1136/jech.2010.123232
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2013) Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age ageing 42(2):203–209. https://doi.org/10.1093/ageing/afs194
Laufer Y (2005) Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. J Gerontol Ser A 60(5):627–632. https://doi.org/10.1093/gerona/60.5.627
Laukkanen P, Heikkinen E, Kauppinen M (1995) Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing 24(6):468–473
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 95(5):1851–1860. https://doi.org/10.1152/japplphysiol.00246.2003
Lebrun CEI, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW (2006) Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause 13(3):474–481. https://doi.org/10.1097/01.gme.0000222331.23478.ec
Manini TM, Clark BC (2012) Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 67(1):28–40. https://doi.org/10.1093/gerona/glr010
Marsh AP, Miller ME, Saikin AM, Rejeski WJ, Hu N, Lauretani F, Bandinelli S, Guralnik JM, Ferrucci L (2006) Lower extremity strength and power are associated with 400-meter walk time in older adults: the InCHIANTI study. J Gerontol Ser A Biol Sci Med Sci 61 (11):1186–1193
Martin HJ, Yule V, Syddall HE, Dennison EM, Cooper C, Aihie Sayer A (2006) Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometry. Gerontology 52(3):154–159
Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, Thabane L, Raina P (2018) The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. https://doi.org/10.1093/ageing/afy106
Mazariegos M, Wang ZM, Gallagher D, Baumgartner RN, Allison DB, Wang J, Pierson RN Jr, Heymsfield SB (1994) Differences between young and old females in the five levels of body composition and their relevance to the two-compartment chemical model. J Gerontol 49(5):M201–M208
Mazess RB, Barden HS, Bisek JP, Hanson J (1990) Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51(6):1106–1112
McGregor RA, Cameron-Smith D, Poppitt SD (2014) It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3:9. https://doi.org/10.1186/2046-2395-3-9
Mitchell W, Atherton P, Williams J, Larvin M, Lund J, Narici M (2012) Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3:260. https://doi.org/10.3389/fphys.2012.00260
Moore AZ, Caturegli G, Metter EJ, Makrogiannis S, Resnick SM, Harris TB, Ferrucci L (2014) Difference in muscle quality over the adult life span and biological correlates in the Baltimore longitudinal study of aging. J Am Geriatr Soc 62(2):230–236. https://doi.org/10.1111/jgs.12653
Nana A, Slater GJ, Hopkins WG, Burke LM (2012) Effects of daily activities on dual-energy X-ray absorptiometry measurements of body composition in active people. Med Sci Sports Exerc 44(1):180–189. https://doi.org/10.1249/MSS.0b013e318228b60e
Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M, The Health A, Body C (2003) Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc 51(3):323–330. https://doi.org/10.1046/j.1532-5415.2003.51105.x
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Rosenberg IH (1989) Summary comments. Am J Clin Nutr 50(5):1231–1233. https://doi.org/10.1093/ajcn/50.5.1231
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39(2):377–390. https://doi.org/10.1249/mss.0b013e31802ca597
Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G (2014) Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int 25 (1):187–193. https://doi.org/10.1007/s00198-013-2431-5
Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djousse L, Lyles MF, Bartz TM, Murthy VL, Shah RV (2016) The association of lean and fat mass with all-cause mortality in older adults: the cardiovascular health study. Nutr Metab Cardiovasc Dis NMCD 26 (11):1039–1047. https://doi.org/10.1016/j.numecd.2016.06.011
Stoever K, Heber A, Eichberg S, Brixius K (2017) Sarcopenia and predictors of skeletal muscle mass in elderly men with and without obesity. Gerontol Geriatr Med. https://doi.org/10.1177/2333721417713637
St-Onge MP, Gallagher D (2010) Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 26(2):152–155. https://doi.org/10.1016/j.nut.2009.07.004
Thurlow S, Oldroyd B, Hind K (2018) Effect of hand positioning on DXA total and regional bone and body composition parameters, precision error, and least significant change. J Clin Densitom Off J Int Soc Clin Densitom 21(3):375–382. https://doi.org/10.1016/j.jocd.2017.03.003
Tieland M, Verdijk LB, de Groot LCPGM, van Loon LJC (2015) Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people. Int J Sport Nutr Exerc Metab 25(1):27–36. https://doi.org/10.1123/ijsnem.2013-0123
Verdijk LB, van Loon L, Meijer K, Savelberg HH (2009) One-repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci 27(1):59–68. https://doi.org/10.1080/02640410802428089
Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50(5):897–904
Yeung SSY, Reijnierse EM, Trappenburg MC, Blauw GJ, Meskers CGM, Maier AB (2018) Knee extension strength measurements should be considered as part of the comprehensive geriatric assessment. BMC Geriatr 18(1):130. https://doi.org/10.1186/s12877-018-0815-2
Acknowledgements
The authors wish to thank the participants for their time and cooperation throughout this study.
Author information
Authors and Affiliations
Contributions
ML, OW, KH and TI contributed towards the research design. ML conducted all experimental procedures. ML and TI performed data analysis. ML, OW, KH and TI wrote the manuscript and all authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lees, M.J., Wilson, O.J., Hind, K. et al. Muscle quality as a complementary prognostic tool in conjunction with sarcopenia assessment in younger and older individuals. Eur J Appl Physiol 119, 1171–1181 (2019). https://doi.org/10.1007/s00421-019-04107-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04107-8