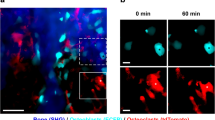
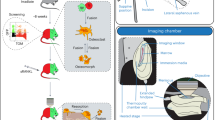
Abstract
There are as many as 200 cell types in the body, and highly sophisticated and varied life phenomena are carried out by cell migration to appropriate places at appropriate times following the appropriate interactions. Recent advances in optical imaging technology using multi-photon excitation microscopy have enabled visualization inside intact bone tissues in living animals without thin sectioning. Using such advanced techniques, the dynamic behaviors of living bone cells on intact bone tissue structures can be elucidated. Here, we focus on recent findings using intravital multi-photon imaging of dynamic biological systems, e.g., bone homeostasis. This novel approach has proven beneficial for understanding the mechanisms underlying the spatiotemporal nature of bone remodeling systems and for evaluating the specific modes of actions of novel drugs currently in development, which will contribute to a new chapter in bone and mineral research.








Similar content being viewed by others
References
Campagnola PJ, Loew LM (2003) Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol 21:1356–1360. https://doi.org/10.1038/nbt894
Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H (2012) Osteoprotection by semaphorin 3A. Nature 485:69–74. https://doi.org/10.1038/nature11000
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458:524–528. https://doi.org/10.1038/nature07713
Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN (2010) Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 207:2793–2798. https://doi.org/10.1084/jem.20101474
Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H, Ishii M (2013a) Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci USA 110:7009–7013. https://doi.org/10.1073/pnas.1218799110
Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I, Mizukami S, Maiya N, Yasuda H, Kumanogoh A, Kikuchi K, Germain RN, Ishii M (2013b) Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest 123(2):866–873. https://doi.org/10.1172/JCI65054
Kowada T, Kikuta J, Kubo A, Ishii M, Maeda H, Mizukami S, Kikuchi K et al (2011) In vivo fluorescence imaging of bone-resorbing osteoclasts. J Am Chem Soc 133:17772–17776. https://doi.org/10.1021/ja2064582
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4894 932. https://doi.org/10.1021/cr078381n
Matsuo K, Irie N (2008) Osteoclast-osteoblast communication. Arch Biochem Biophys 473:201–209. https://doi.org/10.1016/j.abb.2008.03.027
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234. https://doi.org/10.1038/nm.2452
Ohba S, Ikeda T, Kugimiya F, Yano F, Lichtler AC, Nakamura K, Takato T, Kawaguchi H, Chung UI (2007) Identification of a potent combination of osteogenic genes for bone regeneration using embryonic stem (ES) cell-based sensor. FASEB J 21:1777–1787. https://doi.org/10.1096/fj.06-7571com
Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y (2002) Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299:483–487
Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A (2008) Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132:487–498. https://doi.org/10.1016/j.cell.2007.12.033
Sun-Wada GH, Tabata H, Kawamura N, Aoyama M, Wada Y (2009) Direct recruitment of H+-ATPase from lysosomes for phagosomal acidification. J Cell Sci 122(Pt 14):2504–2513. https://doi.org/10.1242/jcs.050443
Wang B-G, König K, Halbhuber K-J (2010) Two-photon microscopy of deep intravital tissues and its merits in clinical research. J Microsc 238:1–20. https://doi.org/10.1111/j.1365-2818.2009.03330.x
Wang X, Chen X, Yang Y (2012) Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 9:266–269. https://doi.org/10.1038/nmeth.1892
Acknowledgements
This work was supported by CREST, Japan Science and Technology Agency, and Grants-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS to M.I.); Grant-in-Aid for Young Scientists (A) from JSPS (to J.K.); and Grant-in-Aid for Young Scientists (B) from JSPS (to H.M.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mizuno, H., Kikuta, J. & Ishii, M. In vivo live imaging of bone cells. Histochem Cell Biol 149, 417–422 (2018). https://doi.org/10.1007/s00418-018-1638-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1638-0