Abstract
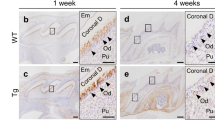
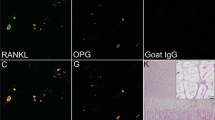
Dmp1 is an acidic phosphoprotein that is specifically expressed in osteocytes. During the secretory process, the full-length, precursor Dmp1 is cleaved into N- and C-terminal fragments. C-terminal Dmp1 is phosphorylated, becoming a highly negatively charged domain that may assist in bone mineralization by recruiting calcium ions and influencing subsequent mineral deposition. It has been recently reported that the Golgi-localized protein kinase Fam20C phosphorylates Dmp1 in vitro. To investigate this phosphorylation in situ, we determined the locations of phosphorylated Dmp1 and Fam20C in rat bones using immunohistochemistry. During osteocytogenesis, osteoblastic, osteoid, and young osteocytes (but not old osteocytes) express Dmp1 mRNA and contain Dmp1 protein in the Golgi apparatus. These Dmp1-producing cells were distributed across the surface layer of cortical bone. Using immunofluorescence, we found that N- and C-terminal Dmp1 fragments were predominantly distributed along the lacunar walls and canaliculi of mineralized bone, respectively, but were not present in the osteoid matrix. We also found that Fam20C and its substrate, C-terminal Dmp1, colocalized in the Golgi of osteoblastic, osteoid, and young osteocytes. Furthermore, phosphorylated C-terminal Dmp1 was present in the Golgi of young osteocytes. Double-labeling immunoelectron microscopy revealed that phosphorylated C-terminal Dmp1 localized to the canalicular wall in mineralized bone. These findings suggest that C-terminal Dmp1 is phosphorylated within osteocytes and then secreted into the pericanalicular matrix of mineralized bone. Phosphorylated, negatively charged C-terminal Dmp1 in the pericanalicular matrix may play an important role in bone mineralization by recruiting calcium ions.









Similar content being viewed by others
References
Bahl JM, Jensen SS, Larsen MR, Heegaard NH (2008) Characterization of the human cerebrospinal fluid phosphoproteome by titanium dioxide affinity chromatography and mass spectrometry. Anal Chem 80:6308–6316
Carrascal M, Gay M, Ovelleiro D, Casas V, Gelpí E, Abian J (2010) Characterization of the human plasma phosphoproteome using linear ion trap mass spectrometry and multiple search engines. J Proteome Res 9:876–884
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS (2001) Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun 280:460–465
Franz-Odendaal TA, Hall BK, Witten PE (2006) Buried alive: how osteoblasts become osteocytes. Dev Dyn 235:176–190
Gajjeraman S, Narayanan K, Hao J, Qin C, George A (2007) Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282:1193–1204
George A, Hao J (2005) Role of phosphophoryn in dentin mineralization. Cells Tissues Organs 181:232–240
Gorski JP (1992) Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int 50:391–396
Hao J, Narayanan K, Muni T, Ramachandran A, George A (2007) Dentin matrix protein 4, a novel secretory calcium-binding protein that modulates odontoblast differentiation. J Biol Chem 282:15357–15365
He G, George A (2004) Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem 279:11649–11656
Huang B, Maciejewska I, Sun Y, Peng T, Qin D, Lu Y, Bonewald L, Butler WT, Feng J, Qin C (2008) Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int 82:401–410
Linde A, Lussi A, Crenshaw MA (1989) Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int 44:286–295
Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ (2011) The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res 26:331–340
MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem 272:835–842
Maciejewska I, Cowan C, Svoboda K, Butler WT, D’Souza R, Qin C (2009) The NH2-terminal and COOH-terminal fragments of dentin matrix protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem 57:155–166
Mikuni-Takagaki Y, Kakai Y, Satoyoshi M, Kawano E, Suzuki Y, Kawase T, Saito S (1995) Matrix mineralization and the differentiation of osteocyte-like cells in culture. J Bone Miner Res 10:231–242
Nefussi JR, Sautier JM, Nicolas V, Forest N (1991) How osteoblasts become osteocytes: a decreasing matrix forming process. J Biol Buccale 19:75–82
Palumbo C, Palazzini S, Zaffe D, Marotti G (1990) Osteocyte differentiation in the tibia of newborn rabbit: an ultrastructural study of the formation of cytoplasmic processes. Acta Anat (Basel) 137:350–358
Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS, Bhown M, Schrohenloher RE (1987) Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem 262:2900–2907
Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT (2003) Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem 278:34700–34708
Qin C, Baba O, Butler WT (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136
Rabie AM, Veis A (1995) An immunocytochemical study of the routes of secretion of collagen and phosphophoryn from odontoblasts into dentin. Connect Tissue Res 31:197–209
Salih E (2003) In vivo and in vitro phosphorylation regions of bone sialoprotein. Connect Tissue Res 44(Supp 1):223–229
Sato S, Hashimoto J, Usami Y, Ohyama K, Isogai Y, Hagiwara Y, Maruyama N, Komori T, Kuroda T, Toyosawa S (2013) Novel sandwich ELISAs for rat DMP1: age-related decrease of circulatory DMP1 levels in male rats. Bone 57:429–436
Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE (2012) Secreted Kinase phosphorylates extracellular proteins that regulate biomineralization. Science 336:1150–1153
Tagliabracci VS, Pinna LA, Dixon JE (2013) Secreted protein kinases. Trends Biochem Sci 38:121–130
Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL (2004) In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem 279:18115–18120
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T (2001) Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res 16:2017–2026
Wang X, Hao J, Xie Y, Sun Y, Hernandez B, Yamoah AK, Prasad M, Zhu Q, Feng JQ, Qin C (2010) Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem 58:957–967
Wang X, Wang S, Lu Y, Gibson MP, Liu Y, Yuan B, Feng JQ, Qin C (2012) FAM20C plays an essential role in the formation of murine teeth. J Biol Chem 287:35934–35942
Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ (2005) DMP1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem 280:6197–6203
Zhou W, Ross MM, Tessitore A, Ornstein D, Vanmeter A, Liotta LA, Petricoin EF 3rd (2009) An initial characterization of the serum phosphoproteome. J Proteome Res 8:5523–5531
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Number 24390409 and 26670801, and was partially supported by “Nanotechnology Platform” (Project No. 12024046) of MEXT. We acknowledge Emeritus Prof. Yoshiro Takano and Dr. Yukiko Nakano who belonged to Tokyo Medical and Dental University for their support with immunohistochemistry, and Dr. Yu Usami of Osaka University for advice regarding the confocal microscope images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Oya, K., Ishida, K., Nishida, T. et al. Immunohistochemical analysis of dentin matrix protein 1 (Dmp1) phosphorylation by Fam20C in bone: implications for the induction of biomineralization. Histochem Cell Biol 147, 341–351 (2017). https://doi.org/10.1007/s00418-016-1490-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1490-z