Abstract
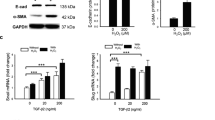
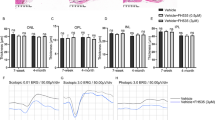
Elevated Notch signaling has been verified in a large range of fibrotic diseases developed in the kidney, liver, and lung, inducing the development of the epithelial–mesenchymal transition (EMT). The aim of this study was to observe the involvement of Notch signaling in the EMT of retinal pigment epithelial (RPE) cells and the pathogenesis of proliferative vitreoretinopathy (PVR). In vitro cultivated human RPE cells (ARPE-19) were treated with 10 ng/mL transforming growth factor (TGF)-β1 for 24, 48, and 72 h. The expression levels of ZO-1, α-SMA, vimentin, Notch1 intracellular domain (NICD1), and Hes-1 were evaluated with quantitative real-time polymerase chain reaction (qRT-PCR), immunofluorescence staining or Western blot. TGF-β1 induced EMT and the activation of Notch signaling in ARPE-19 cells. To examine the effect of Notch inhibition on TGF-β1-induced EMT and PVR formation, ARPE-19 cells were preincubated with γ-secretase inhibitor LY411575 before TGF-β1 treatment. Mouse PVR model was used for in vivo study. ARPE-19 cells were injected intravitreously with or without the LY411575 to examine the effect of Notch inhibition on PVR formation. LY411575 significantly attenuated EMT by inhibiting the Notch signaling activation in vitro. PVR was induced by intravitreal injections of ARPE-19 cells, while LY411575 inhibited mouse PVR formation in vivo. Notch signaling plays a critical role in TGF-β1-induced EMT in vitro and mice PVR model, which provides a novel insight into the pathogenesis of PVR. The specific inhibition of Notch signaling by γ-secretase inhibitor may provide a new approach for the prevention of PVR.




Similar content being viewed by others
References
Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR (2007) In vivo models of proliferative vitreoretinopathy. Nat Protoc 2:67–77
Ahmad I, Balasubramanian S, Del Debbio CB, Parameswaran S, Katz AR, Toris C, Fariss RN (2011) Regulation of ocular angiogenesis by Notch signaling: implications in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 52:2868–2878
Bai Y, Yu W, Han N, Yang F, Sun Y, Zhang L, Zhao M, Huang L, Zhou A, Wang F, Li X (2013) Effects of semaphorin 3A on retinal pigment epithelial cell activity. Invest Ophthalmol Vis Sci 54:6628–6638
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
Chen Y, Zheng S, Qi D, Zheng S, Guo J, Zhang S, Weng Z (2012) Inhibition of Notch signaling by a γ-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS ONE 7:e46512
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y (2014) Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Curr Mol Med 14:523–534
Hills CE, Squires PE (2010) TGF-β1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol 31:68–74
Hiscott P, Sheridan C, Magee RM, Grierson I (1999) Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res 18:167–190
Hoerster R, Muether PS, Vierkotten S, Hermann MM, Kirchhof B, Fauser S (2014) Upregulation of TGF-β1 in experimental proliferative vitreoretinopathy is accompanied by epithelial to mesenchymal transition. Graefes Arch Clin Exp Ophthalmol 252:11–16
Jiang M, Wu PC, Fini ME, Tsai CL, Itakura T, Zhang X, Jiao S (2012) Single-shot dimension measurements of the mouse eye using SD-OCT. Ophthalmic Surg Lasers Imaging 43:252–256
Leask A (2010) Targeting the Jagged/Notch pathway: a new treatment for fibrosis? J Cell Commun Signal 4:197–198
Lei H, Rheaume MA, Cui J, Mukai S, Maberley D, Samad A, Matsubara J, Kazlauskas A (2012) A novel function of p53: a gatekeeper of retinal detachment. Am J Pathol 181:866–874
Li Y, Ma J, Qian X, Wu Q, Xia J, Miele L, Sarkar FH, Wang Z (2013) Regulation of EMT by Notch signaling pathway in tumor progression. Curr Cancer Drug Targets 13:957–962
Liu W, Jin G, Long C, Zhou X, Tang Y, Huang S, Kuang X, Wu L, Zhang Q, Shen H (2013) Blockage of Notch signaling inhibits the migration and proliferation of retinal pigment epithelial cells. Sci World J 2013:178708
Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A (2004) Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res 94:910–917
Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, Karsan A (2006) Smooth muscle α-actin is a direct target of Notch/CSL. Circ Res 98:1468–1470
Ono H, Imoto I, Kozaki K, Tsuda H, Matsui T, Kurasawa Y, Muramatsu T, Sugihara K, Inazawa J (2012) SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene 31:4923–4934
Ou J, Bharti K, Nodari A, Bertuzzi S, Arnheiter H (2013) Vax1/2 genes counteract Mitf-induced respecification of the retinal pigment epithelium. PLoS ONE 8:e59247
Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J (2011) Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 118:3436–3439
Qi X, Cai J, Ruan Q, Liu L, Boye SL, Chen Z, Hauswirth WW, Ryals RC, Shaw L, Caballero S, Grant MB, Boulton ME (2012) γ-Secretase inhibition of murine choroidal neovascularization is associated with reduction of superoxide and proinflammatory cytokines. Invest Ophthalmol Vis Sci 53:574–585
Qiu S, Jiang Z, Huang Z, Chen X, Qian X, Gao Q, Zheng H (2013) Migration of retinal pigment epithelium cells is regulated by protein kinase Cα in vitro. Invest Ophthalmol Vis Sci 54:7082–7090
Soler MVC, Gallo JE, Dodds RA, Suburo AM (2002) A mouse model of proliferative vitreoretinopathy induced by dispase. Exp Eye Res 75:491–504
Tamiya S, Liu L, Kaplan HJ (2010) Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Invest Ophthalmol Vis Sci 51:2755–2763
Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ (2004) Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol 137:1105–1115
Umazume K, Liu L, Scott PA, de Castro JP, McDonald K, Kaplan HJ, Tamiya S (2013) Inhibition of PVR with a tyrosine kinase inhibitor, dasatinib, in the swine. Invest Ophthalmol Vis Sci 54:1150–1159
Wang Z, Li Y, Kong D, Sarkar FH (2010) The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets 11:745–751
Wilson A, Radtke F (2006) Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett 580:2860–2868
Yu J, Liu F, Cui SJ, Liu Y, Song ZY, Cao H, Chen FE, Wang WJ, Sun T, Wang F (2008) Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics 8:3667–3678
Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP (2004) Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23:1155–1165
Zeng F, Zhang M, Xu Y, Xu H (2013) ARMS2 interference leads to decrease of proinflammatory mediators. Graefes Arch Clin Exp 251:2539–2544
Zhang J, Zhou Q, Yuan G, Dong M, Shi W (2015) Notch signaling regulates M2 type macrophage polarization during the development of proliferative vitreoretinopathy. Cell Immunol 298:77–82
Zhu F, Li T, Qiu F, Fan J, Zhou Q, Ding X, Nie J, Yu X (2010) Preventive effect of Notch signaling inhibition by a γ-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am J Pathol 176:650–659
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81470611, 81530027); Shandong Provincial Natural Science Foundation, China (JQ201518, ZR2015PH001), and Youth Foundation of Shandong Academy of Medical Sciences (2014-41). The authors thank Ms. Ping Lin for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Zhang, J., Yuan, G., Dong, M. et al. Notch signaling modulates proliferative vitreoretinopathy via regulating retinal pigment epithelial-to-mesenchymal transition. Histochem Cell Biol 147, 367–375 (2017). https://doi.org/10.1007/s00418-016-1484-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1484-x