Abstract
Purpose
This study investigated the hypotensive effect of RKI-1447, a Rho kinase inhibitor, in a porcine ex vivo pigmentary glaucoma model.
Methods
Twenty-eight porcine anterior chambers were perfused with medium supplemented with 1.67 × 107 pigment particles/ml for 48 h before treatment with RKI-1447 (n = 16) or vehicle control (n = 12). Intraocular pressure (IOP) was recorded and outflow facility was calculated. Primary trabecular meshwork cells were exposed to RKI-1447 or vehicle control; effects on the cytoskeleton, motility, and phagocytosis were evaluated.
Result
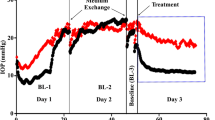
Compared to baseline, the perfusion of pigment caused a significant increase in IOP in the RKI-1447 group (P = 0.003) at 48 h. Subsequent treatment with RKI-1447 significantly reduced IOP from 20.14 ± 2.59 to 13.38 ± 0.91 mmHg (P = 0.02). Pigment perfusion reduced the outflow facility from 0.27 ± 0.03 at baseline to 0.18 ± 0.02 at 48 h (P < 0.001). This was partially reversed with RKI-1447. RKI-1447 caused no apparent histological changes in the micro- or macroscopic TM appearance. RKI-1447-treated primary TM cells showed significant disruption of the actin cytoskeleton both in the presence and absence of pigment (P < 0.001) but no effect on TM migration was observed. Pigment-treated TM cells exhibited a reduction in TM phagocytosis, which RKI-1447 reversed.
Conclusion
RKI-1447 significantly reduces IOP by disrupting TM stress fibers and increasing TM phagocytosis. These features may make it useful for the treatment of secondary glaucomas with an increased phagocytic load.





Similar content being viewed by others
References
Janssen SF, Gorgels TGMF, Ramdas WD et al (2013) The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res 37:31–67
Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311:1901–1911
Weinreb RN, Ong T, Scassellati Sforzolini B et al (2015) A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol 99:738–745
Waxman S, Wang C, Dang Y, Hong Y, Esfandiari H, Shah P, Lathrop KL, Loewen RT, Loewen NA (2018) Structure–function changes of the porcine distal outflow tract in response to nitric oxide. Invest Ophthalmol Vis Sci 1;59(12):4886–4895
McDonnell F, Dismuke WM, Overby DR, Stamer WD (2018) Pharmacological regulation of outflow resistance distal to Schlemm’s canal. Am J Physiol Cell Physiol 315:C44–C51
Garnock-Jones KP (2014) Ripasudil: first global approval. Drugs 74:2211–2215
Tanihara H, Inoue T, Yamamoto T et al (2016) One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol 94:e26–e34
Patel RA, Forinash KD, Pireddu R et al (2012) RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res 72:5025–5034
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4:446–456
Katoh K, Kano Y, Ookawara S (2007) Rho-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes Cells 12:623–638
Wang J, Liu X, Zhong Y (2013) Rho/Rho-associated kinase pathway in glaucoma (Review). Int J Oncol 43:1357–1367
Goldhagen B, Proia AD, Epstein DL, Rao PV (2012) Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. J Glaucoma 21:530–538
National Library of Medicine (2014) Multiple dose-parallel-group study of AMA0076 in patients with primary open-angle glaucoma or ocular hypertensionhttps://clinicaltrials.gov/ct2/show/NCT02136940. Accessed 7 Sep 2017
National Library of Medicine (2016) Double-masked study of PG324 ophthalmic solution in patients with open-angle glaucoma or ocular hypertension. https://clinicaltrials.gov/ct2/show/NCT02674854. Accessed 7 Sep 2017
Bacharach J, Dubiner HB, Levy B et al (2015) Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology 122:302–307
Choy M (2018) Pharmaceutical approval update. P T 43:205–227
Tanihara H, Inoue T, Yamamoto T et al (2015) Additive intraocular pressure-lowering effects of the Rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol 133:755–761
Wang SK, Chang RT (2014) An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin Ophthalmol 8:883–890
Martin MP, Zhu J-Y, Schonbrunn E (2012) Rho-associated protein kinase 1 (ROCK 1) IN COMPLEX WITH RKI1447. https://doi.org/10.2210/pdb3TWJ/pdb
Dang Y, Waxman S, Wang C et al (2018) A porcine ex vivo model of pigmentary glaucoma. Sci Rep 8:5468
McMenamin PG, Steptoe RJ (1991) Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J Anat 178:65–77
Ruiz-Ederra J, García M, Hernández M et al (2005) The pig eye as a novel model of glaucoma. Exp Eye Res 81:561–569
Loewen RT, Roy P, Park DB et al (2016) A porcine anterior segment perfusion and transduction model with direct visualization of the trabecular meshwork. Invest Ophthalmol Vis Sci 57:1338–1344
Dang Y, Waxman S, Wang C et al (2017) Freeze-thaw decellularization of the trabecular meshwork in an ex vivo eye perfusion model. PeerJ 5:e3629
Tian B, Hu Y, Gabelt BT, Kaufman PL (2006) Factors affecting outflow facility calculations. Exp Eye Res 83:1515–1520
Dang Y, Kaplowitz K, Parikh HA et al (2016) Steroid-induced glaucoma treated with trabecular ablation in a matched comparison with primary open-angle glaucoma. Clin Exp Ophthalmol 44:783–788
Wang G-Q, Dang Y-L, Huang Q et al (2017) In vitro evaluation of the effects of intraocular lens material on lens epithelial cell proliferation, migration, and transformation. Curr Eye Res 42:72–78
Fan Q, Teo Y-Y, Saw S-M (2011) Application of advanced statistics in ophthalmology. Invest Ophthalmol Vis Sci 52:6059–6065
R Development Core Team (2015) R: a language and environment for statistical computing. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. Accessed 7 Sep 2018
Hogg P, Calthorpe M, Batterbury M, Grierson I (2000) Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci 41:1091–1098
Siddiqui Y, Ten Hulzen RD, Cameron JD et al (2003) What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol 135:794–799
Ritch R, Steinberger D, Liebmann JM (1993) Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am J Ophthalmol 115:707–710
Qing G, Wang N, Tang X et al (2009) Clinical characteristics of pigment dispersion syndrome in Chinese patients. Eye 23:1641–1646
Llobet A, Gasull X, Gual A (2003) Understanding trabecular meshwork physiology: a key to the control of intraocular pressure? News Physiol Sci 18:205–209
Abu-Hassan DW, Acott TS, Kelley MJ (2014) The trabecular meshwork: a basic review of form and function. J Ocul Biol Dis Infor 2:1–22
Nakajima E, Nakajima T, Minagawa Y et al (2005) Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J Pharm Sci 94:701–708
Doornaert B, Leblond V, Planus E et al (2003) Time course of actin cytoskeleton stiffness and matrix adhesion molecules in human bronchial epithelial cell cultures. Exp Cell Res 287:199–208
Wang K, Read AT, Sulchek T, Ethier CR (2017) Trabecular meshwork stiffness in glaucoma. Exp Eye Res 158:3–12
Calzado-Martín A, Encinar M, Tamayo J et al (2016) Effect of actin organization on the stiffness of living breast cancer cells revealed by peak-force modulation atomic force microscopy. ACS Nano 10:3365–3374
Raghunathan VK, Morgan JT, Park SA et al (2015) Dexamethasone stiffens trabecular meshwork, trabecular meshwork cells, and matrix. Invest Ophthalmol Vis Sci 56:4447–4459
Gavara N, Chadwick RS (2016) Relationship between cell stiffness and stress fiber amount, assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech Model Mechanobiol 15:511–523
Morgan JT, Raghunathan VK, Chang Y-R et al (2015) The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 6:15362–15374
Peng J, Feng X-Y, Ye Z-M et al (2016) Effects of dexamethasone and HA1077 on actin cytoskeleton and β-catenin in cultured human trabecular meshwork cells. Int J Ophthalmol 9:1376–1380
Clark AF, Brotchie D, Read AT et al (2005) Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton 60:83–95
Rao PV, Deng PF, Kumar J, Epstein DL (2001) Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci 42:1029–1037
Rao PV, Deng P, Sasaki Y, Epstein DL (2005) Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res 80:197–206
Zhang M, Rao PV (2005) Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci 46:4130–4138
Honjo M, Inatani M, Kido N et al (2002) A myosin light chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp Eye Res 75:135–142
Honjo M, Tanihara H, Inatani M et al (2001) Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci 42:137–144
Rao PV, Deng P, Maddala R et al (2005) Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis 11:288–297
May RC, Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114:1061–1077
Mannherz HG (2017) The actin cytoskeleton and bacterial infection. Springer, New York City
Rohen JW, van der Zypen E (1968) The phagocytic activity of the trabecular meshwork endothelium. Graefes Arch Clin Exp Ophthalmol 175:143–160
Matsumoto Y, Johnson DH (1997) Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci 38:1902–1907
Castellano F, Le Clainche C, Patin D et al (2001) A WASp–VASP complex regulates actin polymerization at the plasma membrane. EMBO J 20:5603–5614
Camras LJ, Stamer WD, Epstein D et al (2012) Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Invest Ophthalmol Vis Sci 53:5242–5250
Loewen RT, Brown EN, Scott G et al (2016) Quantification of focal outflow enhancement using differential canalograms. Invest Ophthalmol Vis Sci 57:2831–2838
Loewen RT, Brown EN, Roy P et al (2016) Regionally discrete aqueous humor outflow quantification using fluorescein canalograms. PLoS One 11:e0151754
Parikh HA, Loewen RT, Roy P et al (2016) Differential canalograms detect outflow changes from trabecular micro-bypass stents and ab interno trabeculectomy. Sci Rep 6:34705
Funding
We acknowledge support from K08-EY022737 (NAL), from NIH CORE Grant P30 EY08098 to the Department of Ophthalmology, from the Initiative to Cure Glaucoma of the Eye and Ear Foundation of Pittsburgh (NAL), the Wiegand Fellowship (YD), and an unrestricted grant from the Research to Prevent Blindness, New York, NY, an unrestricted grant from the Third Xiangya Hospital of Central South University for studying at the University of Pittsburgh (CW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. No animals were sacrificed for the purpose of doing research. An approval by an ethics committee or Institutional Animal Care and Use Committee was not required.
Rights and permissions
About this article
Cite this article
Dang, Y., Wang, C., Shah, P. et al. RKI-1447, a Rho kinase inhibitor, causes ocular hypotension, actin stress fiber disruption, and increased phagocytosis. Graefes Arch Clin Exp Ophthalmol 257, 101–109 (2019). https://doi.org/10.1007/s00417-018-4175-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4175-6