Abstract
Objective
To report a kindred with an association between hereditary primary lateral sclerosis (PLS) and progressive nonfluent aphasia.
Patients and methods
Six members from a kindred with 15 affected individuals spanning three generations, suffered from spasticity without muscle atrophy or fasciculation, starting in the lower limbs and spreading to the upper limbs and bulbar musculature, followed by effortful speech, nonfluent language and dementia, in 5 deceased members. Disease onset was during the sixth decade of life, or later. Cerebellar ataxia was the inaugural manifestation in two patients, and parkinsonism, in another.
Results
Neuropathological examination in two patients demonstrated degeneration of lateral corticospinal tracts in the spinal cord, without loss of spinal, brainstem, or cerebral motor neurons. Greater loss of corticospinal fibers at sacral and lumbar, rather than at cervical or medullary levels was demonstrated, supporting a central axonal dying-back pathogenic mechanism. Marked reduction of myelin and nerve fibers in the frontal lobes was also present. Argyrophilic grain disease and primary age-related tauopathy were found in one case each, and considered incidental findings. Genetic testing, including exome sequencing aimed at PLS, ataxia, hereditary spastic paraplegia, and frontotemporal lobe dementia, triplet-repeated primed polymerase chain reaction aimed at dominant spinocerebellar ataxias, and massive sequencing of the human genome, yielded negative results.
Conclusion
A central distal axonopathy affecting the corticospinal tract, exerted a pathogenic role in the dominantly inherited PLS-progressive nonfluent aphasia association, described herein. Further molecular studies are needed to identify the causative mutation in this disease.





Similar content being viewed by others
References
Agarwal S, Highton-Williamson E, Caga J, Matamala JM, Dharmadasa T, Howells J et al (2018) Primary lateral sclerosis and the amyotrophic lateral sclerosis-frontotemporal dementia spectrum. J Neurol 265:1819–1828
Agosta F, Galantucci S, Magnani G, Marcone A, Martinelli D, Antonietta Volontè M et al (2015) MRI signatures of the frontotemporal lobar degeneration continuum. Hum Brain Mapp 36:2602–2614
Besser LM, Crary JF, Mock C, Kukull WA (2017) Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 89:1707–1715
Brugman F, Wokke JH, Vianney de Jong JM, Franssen H, Faber CG, Van den Berg LH (2005) Primary lateral sclerosis as a phenotypic manifestation of familial ALS. Neurology 64:1778–1779
Brugman F, Eymard-Pierre E, van den Berg LH, Wokke JH, Gauthier-Barichard F, Boespflug-Tanguy O (2007) Adult-onset primary lateral sclerosis is not associated with mutations in the ALS2 gene. Neurology 69:702–704
Brugman F, Veldink JH, Franssen H, de Visser M, de Jong JM, Faber CG et al (2009) Differentiation of hereditary spastic paraparesis from primary lateral sclerosis in sporadic adult-onset upper motor neuron syndromes. Arch Neurol 66:509–514
Canu E, Agosta F, Galantucci S, Chiò A, Riva N, Silani V et al (2013) Extramotor damage is associated with cognition in primary lateral sclerosis patients. PLoS One 8:e82017
Caselli RJ, Smith BE, Osborne D (1995) Primary lateral sclerosis: a neuropsychological study. Neurology 45:2005–2009
Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ et al (2014) New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 85:865–870
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766
Deng HX, Zhai H, Fu R, Shi Y, Gorrie GH, Yang Y et al (2007) Distal axonopathy in an alsin-deficient mouse model. Hum Mol Genet 16:2911–2920
de Vries BS, Rustemeijer LMM, van der Kooi AJ, Raaphorst J, Schröder CD, Nijboer TCW et al (2017) A case series of PLS patients with frontotemporal dementia and overview of the literature. Amyotroph Lateral Scler Frontotemporal Degener 18:534–548
de Vries BS, Rustemeijer LMM, Bakker LA, Schröder CD, Veldink JH, van den Berg LH et al (2019) Cognitive and behavioural changes in PLS and PMA: challenging the concept of restricted phenotypes. J Neurol Neurosurg Psychiatry 90:141–147
Doran M, Enevoldson TP, Ghadiali EJ, Larner AJ (2005) Mills syndrome with dementia: broadening the phenotype of FTD/MND. J Neurol 252:846–847
Dupré N, Valdmanis PN, Bouchard JP, Rouleau GA. Autosomal dominant primary lateral sclerosis. Neurology 68:1156–7
Elahi FM, Miller BL (2017) A clinicopathologic approach to the diagnosis of dementia. Nat Rev Neurol 13:457–476
Engel PA, Grunnet M (2000) Atypical dementia and spastic paraplegia in a patient with primary lateral sclerosis and numerous neocortical beta amyloid plaques: new disorder or Alzheimer’s disease variant? J Geriatr Psychiatry Neurol 13:60–64
Erb W (1902) Spastic and syphilitic spinal paralysis. Lancet 2:969–974
Folstein M, Folstein SE, McHugh PR (1975) “Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gilbert RM, Fahn S, Mitsumoto H, Rowland LP (2010) Parkinsonism and motor neuron diseases: twenty-seven patients with diverse overlap syndromes. Mov Disord 25:1868–1875
Gómez-Tortosa E, Van der Zee J, Ruggiero M, Gijselinck I, Esteban-Pérez J, García-Redondo A et al (2017) Familial primary lateral sclerosis or dementia associated with Arg573Gly TBK1 mutation. J Neurol Neurosurg Psychiatry 88:996–997
Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, Rowland LP (2006) The natural history of primary lateral sclerosis. Neurology 66:647–653
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014
Haugh AB, Pandyan AD, Johnson GR (2006) A systematic review of the Tardieu Scale for the measurement of spasticity. Disabil Rehabil 28:899–907
Helal M, Mazaheri N, Shalbafan B, Malamiri RA, Dilaver N, Buchert R et al (2018) Clinical presentation and natural history of infantile-onset ascending spastic paralysis from three families with an ALS2 founder variant. Neurol Sci 39:1917–1925
Hudson AJ, Kiernan JA, Muñoz DG, Pringle CE, Brown WF, Ebers GC (1993) Clinicopathological features of primary lateral sclerosis are different from amyotrophic lateral sclerosis. Brain Res Bull 30:359–364
Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E et al (2011) White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 134:2642–2655
Josephs KA, Knopman DS, Whitwell JL, Boeve BF, Parisi JE, Petersen RC et al (2005) Survival in two variants of tau-negative frontotemporal degeneration: FTLD-U vs. FTLD-MND. Neurology 65:645–647
Josephs KA, Dickson DW (2007) Frontotemporal lobar degeneration with upper motor neuron disease/primary lateral sclerosis. Neurology 69:1800–1801
Josephs KA, Duffy JR (2008) Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol 21:688–692
Josephs KA (2017) Current understanding of neurodegenerative diseases associated with the protein tau. Mayo Clin Proc 92:1291–1303
Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM et al (2017) Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133:705–715
Knopman DS, Nestor PJ (2017) Beyond clinical syndromes in primary progressive aphasia. Seeking etiologic diagnoses. Neurology 88:2244–2245
Kobayashi Z, Tsuchiya K, Arai T, Yokota O, Yoshida M, Shimomura Y et al (2010) Clinicopathological characteristics of FTLD-TDP showing corticospinal tract degeneration but lacking lower motor neuron loss. J Neurol Sci 298:70–77
Kolind S, Sharma R, Knight S, Johansen-Berg H, Talbot K, Turner MR (2013) Myelin imaging in amyotrophic and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 14:562–573
Konagaya M, Sakai M, Matsuoka Y, Konagaya Y, Hashizume Y (1998) Upper motor neuron predominant degeneration with frontal and temporal lobe atrophy. Acta Neuropathol 96:532–536
Kosaka T, Fu YJ, Shiga A, Ishidaira H, Tan CF, Tani T et al (2012) Primary lateral sclerosis: upper-motor-predominant amyotrophic lateral sclerosis with frontotemporal lobar degeneration–immunohistochemical and biochemical analyses of TDP-43. Neuropathology 32:373–384
Lamarche JB, Lemieux B, Lieu HB. The neuropathology of “typical” Friedreich’s ataxia in Quebec (1984). Can J Neurol Sci 11(4 Suppl): 592–600
Le Forestier N, Maisonobe T, Piquard A, Rivaud S, Crevier-Buchman L, Salachas F et al (2001) Does primary lateral sclerosis exist? A study of 20 patients and a review of the literature. Brain 124:1989–1999
Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, Robberecht W et al (2015) A revision of El escorial criteria. Amyotroph Lateral Scler Frontotemporal Degener 16:291–292
Mabuchi N, Watanabe H, Atsuta N, Hirayama M, Ito H, Fukatsu H et al (2004) Primary lateral sclerosis presenting parkinsonian symptoms without nigrostriatal involvement. J Neurol Neurosurg Psychiatry 75:1768–1771
Mackenzie IR, Feldman H (2004) Neurofilament inclusion body disease with early onset frontotemporal dementia and primary lateral sclerosis. Clin Neuropathol 23:183–193
Matilla-Dueñas A, Goold R, Giunti P (2008) Clinical, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum 7:106–114
Medical Research Council (1976) Aids to the examination of the peripheral nervous system. Her Majesty’s Stationery Office, London
Meoded A, Kwan JY, Peters TL, Huey ED, Danielian LE, Wiggs E et al (2013) Imaging findings associated with cognitive performance in primary lateral sclerosis and amyotrophic lateral sclerosis. Dement Geriatr Cogn Dis Extra 3:233–250
Mintchev N, Zamba-Papanicolaou E, Kleopa KA, Christodoulou K (2009) A novel ALS2 splice-site mutation in a Cypriot juvenile-onset primary lateral sclerosis family. Neurology 72:28–32
Mitsumoto H, Nagy PL, Gennings C, Murphy J, Andrews H, Goetz R et al (2015) Phenotypic and molecular analyses of primary lateral sclerosis. Neurol Genet 1:e3
Mochizuki A, Komatsuzaki Y, Iwamoto H, Shoji S (2004) Frontotemporal dementia with ubiquitinated neuronal inclusions presenting with primary lateral sclerosis and parkinsonism: clinicopathological report of an autopsy case. Acta Neuropathol 107:377–380
Murphy MJ, Grace GM, Tartaglia MC, Orange JB, Chen X, Rowe A et al (2008) Cerebral haemodynamic changes accompanying cognitive impairment in primary lateral sclerosis. Amyotroph Lateral Scler 9:359–368
Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H (1999) A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil 13:373–383
Panzeri C, De Palma C, Martinuzzi A, Daga A, De Polo G, Bresolin N et al (2006) The first ALS2 missense mutation associated with JPLS reveals new aspects of alsin biological function. Brain 129:1710–1719
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B et al (1989) Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320:1517–1521
Piquard A, Le Forestier N, Baudoin-Madec V, Delgadillo D, Salachas F, Pradat PF et al (2006) Neuropsychological changes in patients with primary lateral sclerosis. Amyotroph Lat Scler 7:150–160
Polvikoski TM, Murray A, Harper PS, Neal JW (2003) Familial motor neuron disease with dementia: phenotypic variation and cerebellar pathology. J Neurol Neurosurg Psichiatry 74:1516–1520
Praline J, Guennoc AM, Vourc’h P, De Toffol B, Corcia P (2010) Primary lateral sclerosis may occur within familial amyotrophic lateral sclerosis pedigrees. Amyotroph Lat Scler 11:154–156
Pringle CE, Hudson AJ, Muñoz DG, Kiernan JA, Brown WF, Ebers GC (1992) Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 115:495–520
Rodriguez RD, Suemoto CK, Molina M, Nascimento CF, Leite RE, de Lucena Ferretti-Rebustini RE, et al (2016) Argyrophilic grain disease: demographics, clinical, and neuropathological features from a large autopsy study. J Neuropathol Exp Neurol 75:628–635
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Russo LS (1982) Clinical and electrophysiological studies in primary lateral sclerosis. Arch Neurol 39:662–664
Schwindt GC, Graham NL, Rochon E, Tang-Wai DF, Lobaugh NJ, Chow TW et al (2013) Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum Brain Mapp 34:973–984
Schut JW, Haymaker W (1951) Hereditary ataxia. A pathologic study of five cases of common ancestry. J Neuropathol Clin Neurol 1:183–213
Singer MA, Statland JM, Wolfe GI, Barohn RJ (2007) Primary lateral sclerosis. Muscle Nerve 35:291–302
Smith CD (2002) Serial findings in a case of primary lateral sclerosis. Neurology 57:647–649
Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J et al (2017) Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18:153–174
Sugihara H, Horiuchi M, Kamo T, Fujisawa K, Abe M, Sakiyama T et al (1999) A case of primary lateral sclerosis taking a prolonged clinical course with dementia and having an unusual dendritic ballooning. Neuropathology 19:77–84
Tan CF, Kakita A, Piao YS, Kikugawa K, Endo K, Tanaka M et al (2003) Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol 105:615–620
Tartaglia MC, Laluz V, Rowe A, Findlater K, Lee DH, Kennedy K et al (2009) Brain atrophy in primary lateral sclerosis. Neurology 72:1236–1241
Thomas PK, Schaumburg HH, Spencer PS, Kaeser HE, Pallis CA, Rose FC et al (1984) Central distal axonopathy syndromes: newly recognized models of naturally occurring human degenerative disease. Ann Neurol 15:313–315
Valdmanis PN, Dupré N, Rouleau GA (2008) A locus for primary lateral sclerosis on chromosome 4ptel-4p16.1. Arch Neurol 65:383–386
Van Gaalen J, Giunti P, van de Warrenburg BP (2011) Movement disorders in spinocerebellar ataxias. Mov Disord 26:792–800
Vázquez-Costa JF, Bataller L, Vílchez JJ (2016) Primary lateral sclerosis and hereditary spastic paraplegia in sporadic patients. An important distinction in descriptive studies. Ann Neurol 80:169–170
Yamashita S, Ando Y (2015) Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener 3:13
Yang Y, Zhang L, Lynch DR, Lukas T, Ahmeti K, Sleiman PM et al (2016) Compound heterozygote mutations in SPG7 in a family with adult-onset primary lateral sclerosis. Neurol Genet 2:e60
Zhai P, Pagan F, Statland J, Butman JA, Floeter MK (2003) Primary lateral sclerosis. A heterogeneous disorder composed of different subtypes? Neurology 60:1258–1265
Acknowledgements
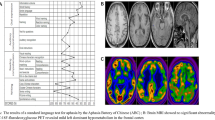
Our thanks to Dr. Antonio Oliveros-Juste for remission of patients; Drs. Ana Vela and Jordi Aldomá for MR images; Mr. Juan Ramón Solans, Drs. Ariadna Fernández-Sanz and Jesús Aladrén-Sangrós for help with the videtapes; Mrs. María Jesús Chopo for obtention of DNA samples; Mrs. Teresa Sopeña Biarge and Mrs. Mar González-Cantalejo from the Medical Library of Hospital Universitario Miguel Servet, for bibliographical research; and Dr. Jorge Alfaro for assistance in the pathological studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical standards
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 15897 KB)
Supplementary material 2 (MP4 34972 KB)
Rights and permissions
About this article
Cite this article
Gazulla, J., Ferrer, I., Izquierdo-Alvarez, S. et al. Hereditary primary lateral sclerosis and progressive nonfluent aphasia. J Neurol 266, 1079–1090 (2019). https://doi.org/10.1007/s00415-019-09235-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09235-x