Abstract
Background
Patients with multiple sclerosis who experience disease breakthrough often switch disease-modifying therapy (DMT).
Objective
To compare treatment effectiveness of switch to highly effective DMT (heDMT) with switch to moderately effective DMT (meDMT) for patients who switch due to disease breakthrough defined as at least one relapse within 12 months of their treatment switch.
Methods
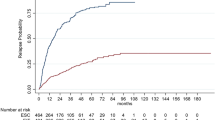
We retrieved data from The Danish Multiple Sclerosis Registry on all relapsing-remitting MS patients with expanded disability status scale (EDSS) less than 6 who experienced disease breakthrough. We used propensity score matching to compare annualized relapse rates (ARRs), time to first confirmed relapse, time to first confirmed EDSS worsening and time to first confirmed EDSS improvement.
Results
Each matched group comprised 404 patients. Median follow-up time was 3.2 years [interquartile range (IQR) 1.7–5.8]. ARRs were 0.22 (0.19–0.27) with heDMT and 0.32 (IQR 0.28–0.37) with meDMT; relapse rate ratio was 0.70 (95% CI 0.56–0.86; p = 0.001). Escalation to heDMT reduced the hazard of reaching a first relapse (HR 0.65; 95% CI 0.53–0.80; p < 0.001). We found no evidence of delayed disability worsening (HR 0.83; 95% CI 0.62–1.10; p = 0.20) and weak evidence of disability improvement (HR 1.33; 95% CI 1.00–1.76; p = 0.05) with heDMT.
Conclusion
Switching to heDMT is associated with reduced ARR and delay of first relapse compared with switching to meDMT. Patients on DMT who experience relapses should escalate therapy to heDMT.



Similar content being viewed by others
References
Sorensen PS, Blinkenberg M (2016) The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord 9:44–52
Sorensen PS (2014) New management algorithms in multiple sclerosis. Curr Opin Neurol 27:246–259
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 43:655–661
Johnson KP, Brooks BR, Cohen JA (1995) Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 45:1268–1276
Jacobs LD, Cookfair DL, Rudick RA et al (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39:285–294
Group P (1998) (Prevention of R and D by I β-1a S in MSS. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352:1498–1504
O’Connor P, Wolinsky JS, Confavreux C et al (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303
Confavreux C, O’Connor P, Comi G et al (2014) Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:247–256
Fox RJ, Miller DH, Phillips JT et al (2012) Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097
Bar-Or A, Gold R, Kappos L et al (2013) Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: Subgroup analyses of the DEFINE study. J Neurol 260:2297–2305
Polman CH, O’Connor PW, Havrdova E et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Calabresi PA, Radue E-W, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556
Kappos L, Radue E-W, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Radue EW, Stuart WH, Calabresi PA et al (2010) Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci 292:28–35
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380:1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839
Caon C, Din M, Ching W et al (2006) Clinical course after change of immunomodulating therapy in relapsing—remitting multiple sclerosis. Eur J Neurol 13:471–474
Gajofatto A, Bacchetti P, Grimes B et al (2009) Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler 15:50–58
Vartanian TK, Zamvil SS, Fox E et al. Neutralizing antibodies to disease-modifying agents in the treatment of multiple sclerosis. Neurology 63:S42–S49
Koch-Henriksen N, Sorensen PS, Bendtzen K et al (2009) The clinical effect of neutralizing antibodies against interferon-beta is independent of the type of interferon-beta used for patients with relapsing-remitting multiple sclerosis. Mult Scler 15:601–605
Rudick RA, Stuart WH, Calabresi PA et al (2006) Natalizumab plus interferon Beta-1a for relapsing multiple sclerosis. N Engl J Med 354:911–923
Soelberg Sorensen P (2016) Safety concerns and risk management of multiple sclerosis therapies. Acta Neurol Scand. https://doi.org/10.1111/ane.12712 (Epub ahead of print)
Carrá A, Onaha P, Luetic G et al (2008) Therapeutic outcome 3 years after switching of immunomodulatory therapies in patients with relapsing-remitting multiple sclerosis in Argentina. Eur J Neurol 15:386–393
He A, Spelman T, Jokubaitis V et al (2015) Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. JAMA Neurol 72:405–413
Spelman T, Kalincik T, Zhang A et al (2015) Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol 2:373–387
Koch-Henriksen N, Magyari M, Sellebjerg F et al (2017) A comparison of multiple sclerosis clinical disease activity between patients treated with natalizumab and fingolimod. Mult Scler J 23:234–241
Braune S, Lang M, Bergmann A (2016) Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 263:327–333
D’Amico E, Leone C, Zanghì A et al (2016) Lateral and escalation therapy in relapsing-remitting multiple sclerosis: a comparative study. J Neurol 263:1802–1809
Gajofatto A, Benedetti MD (2015) Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J Clin Cases 3:545–556
Merkel B, Butzkueven H, Traboulsee AL et al (2017) Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev 16:658–665
Montalban X, Gold R, Thompson AJ et al (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 24:1–23
Acknowledgements
We would like to acknowledge all neurology departments in Denmark for collecting data for The Danish Multiple Sclerosis Registry. Finn Sellebjerg (Chair): Copenhagen University Hospital, Rigshospitalet, Department of Neurology, Copenhagen, Denmark, Email: finn.thorup.sellebjerg@regionh.dk; Melinda Magyari (Secretary): Copenhagen University Hospital, Rigshospitalet, Department of Neurology, Copenhagen, Denmark, Email: melinda.magyari.01@regionh.dk; Morten Blinkenberg: Copenhagen University Hospital, Rigshospitalet, Department of Neurology, Copenhagen, Denmark, Email: blink@dadlnet.dk; Annette Oturai: Copenhagen University Hospital, Rigshospitalet, Department of Neurology, Copenhagen, Denmark, Email: annette.oturai@regionh.dk; Jette Lautrup Frederiksen: Copenhagen University Hospital, Rigshospitalet Glostrup, Department of Neurology, Glostrup, Denmark, Email: jette.lautrup.battistini@regionh.dk; Alex Heick: Copenhagen University Hospital, Rigshospitalet Glostrup, Department of Neurology, Glostrup, Denmark, Email: alexheick@dadlnet.dk; Michael Broksgaard Jensen: North Zealand Hospital, Department of Neurology, Hilleroed, Denmark, Email: michael.broksgaard.jensen@regionh.dk; Lars Stohr: Zealand University Hospital, Roskilde, Department of Neurology, Roskilde, Denmark, Email: laks@regionsjaelland.dk; Monika Góra: Slagelse Hospital, Department of Neurology, Slagelse, Denmark, Email: mkg@regionsjaelland.dk; Helle Hvilsted Nielsen: Odense University Hospital, Department of Neurology, Odense, Denmark, Email: Helle.Hvilsted.Nielsen@rsyd.dk; Zsolt Illes: Odense University Hospital, Department of Neurology, Odense, Denmark, Email: zsolt.illes@rsyd.dk; Mathias Kant: Hospital of Southern Jutland, Department of Neurology, Soenderborg, Denmark, Email: Matthias.Kant1@rsyd.dk; Egon Stenager: Hospital of Southern Jutland, Department of Neurology, Soenderborg, Denmark, Email: egon.stenager3@rsyd.dk; Allan Thimsen Pedersen: Hospital South West Jutland, Department of Neurology, Esbjerg, Denmark, Email: allan.thimsen.pedersen@rsyd.dk; Henrik Boye Jensen: Kolding Hospital, Department of Neurology, Kolding, Denmark, Email: Henrik.Boye.Jensen@rsyd.dk; Thor Petersen: Aarhus University Hospital, Department of Neurology, Aarhus, Denmark, Email: thorpete@rm.dk; Peter Vestergaard Rasmussen: Aarhus University Hospital, Department of Neurology, Aarhus, Denmark, Email: peter.v.rasmussen@aarhus.rm.dk; Lene Rosendahl: Regional Hospital Central Jutland, Department of Neurology, Viborg, Denmark, Email: lene.rosendahl@viborg.rm.dk; Jesper Tørring: Regional Hospital West Jutland, Department of Neurology, Holstebro, Denmark, email: jesper.toerring@vest.rm.dk; Claudia Christina Pfleger: Aalborg University Hospital, Department of Neurology, Aalborg, Denmark, Email: ccp@rn.dk; Arkadiusz Weglewski: Herlev Hospital, Department of Neurology, Herlev, Denmark, Email: arkadiusz.weglewski@regionh.dk
Funding
This study was supported by The Danish Multiple Sclerosis Society; Foundation for Research in Neurology; Gangstedfonden; Ejnar Jonasson called Johnsen and wife’s memorial fund.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
TC has received support for congress participation from Merck, Novartis, Biogen and Roche. TK served on scientific advisory boards for Roche, Genzyme-Sanofi, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Novartis, Biogen, Genzyme-Sanofi, Teva, BioCSL and Merck and received research support from Biogen. BL has nothing to disclose. PS has received personal compensation for serving on advisory boards for Biogen, Merck, Novartis, Teva, MedDay Pharmaceuticals and GSK; on steering committees or independent data monitoring boards in trials sponsored by Merck, Teva, GSK, and Novartis; and has received speaker honoraria from Biogen, Merck Serono, Teva, Sanofi-Aventis, Genzyme, and Novartis. MM has served on scientific advisory board for Biogen Idec, Novartis, Merck, Sanofi and Teva; has received honoraria for lecturing from Biogen Idec, Merck, Novartis and Genzyme; has received support for congress participation from Biogen Idec, Novartis, Genzyme and Teva.
Additional information
Members of the Danish Multiple Sclerosis Group are given in the “Acknowledgements”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chalmer, T.A., Kalincik, T., Laursen, B. et al. Treatment escalation leads to fewer relapses compared with switching to another moderately effective therapy. J Neurol 266, 306–315 (2019). https://doi.org/10.1007/s00415-018-9126-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9126-y