Abstract
Background and aim
Optic neuritis (ON) is a frequent manifestation of multiple sclerosis (MS), traditionally diagnosed clinically and by visually evoked potentials (VEP). However, ON can also be assessed by MRI. Here we compare the diagnostic performance of 3D-double inversion recovery-MRI (3D-DIR) and VEPs in patients with definite MS or clinically isolated syndrome (CIS).
Methods
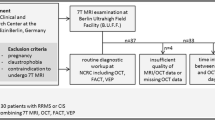
39 patients and 17 healthy controls were studied. Whole-brain-3D-DIR images (3T) were independently assessed for DIR-hyperintense optic nerve lesions (DHLs) by two neuroradiologists, and results related to quantitative VEP-parameters.
Results
Interrater concordance for DHLs was high (κ = 0.82). No DHLs were observed in controls. In patients, abnormal VEPs, i.e. prolonged latencies, diminished amplitudes or abnormal latency or amplitude differences (re contralateral nerve) of the P100-component, were observed in 22, and DHLs in 32 of 78 optic nerves, the latter including 11 nerves with normal VEPs, 10 without clinical signs or history of ON, and 6 with both normal VEPs and no clinical evidence for ON. Using either abnormal VEPs and/or presence of DHLs and/or clinical evidence for ON as a compound reference criterion of optic nerve affection, sensitivity was significantly higher for 3D-DIR than for VEPs (91%, 95%-CI 77–98% vs. 63%, 95%-CI 45–79%, respectively, p = 0.006).
Conclusion
DHLs are highly specific for optic nerve pathology. In the context of MS, 3D-DIR-MRI is a suitable tool to reveal acute or chronic optic nerve lesions and more sensitive than VEPs. The significance of optic nerve involvement in the diagnostic classification of CIS vs. definite MS requires further study.



Similar content being viewed by others
References
Wikstrom J, Poser S, Ritter G (1980) Optic neuritis as an initial symptom in multiple sclerosis. Acta Neurol Scand 61(3):178–185
Halliday AM, McDonald WI, Mushin J (1972) Delayed visual evoked response in optic neuritis. Lancet 1(7758):982–985
American Clinical Neurophysiology S (2006) Guideline 9B: guidelines on visual evoked potentials. Am J Electroneurodiagn Technol 46(3):254–274
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP et al (2010) ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 120(1):111–119. https://doi.org/10.1007/s10633-009-9195-4
Tartaglione A, Oneto A, Bandini F, Spadavecchia L, Gandolfo E, Favale E (1987) Electrophysiological detection of “silent” plaques in the optic pathways. Acta Neurol Scand 76(4):246–250
Balnyte R, Uloziene I, Rastenyte D, Vaitkus A, Malciene L, Lauckaite K (2011) Diagnostic value of conventional visual evoked potentials applied to patients with multiple sclerosis. Medicina (Kaunas) 47(5):263–269
Onofrj M, Tartaro A, Thomas A, Gambi D, Fulgente T, Delli Pizzi C et al (1996) Long echo time STIR sequence MRI of optic nerves in optic neuritis. Neuroradiology 38(1):66–69
Redpath TW (1994) SFW. Imaging gray brain matter with a double-inversion pulse sequence to suppress CSF and white matter signals. MAGMA 2:451–455
Redpath TW, Smith FW (1994) Technical note: use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol 67(804):1258–1263
Wattjes MP, Lutterbey GG, Gieseke J, Traber F, Klotz L, Schmidt S et al (2007) Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 28(1):54–59
Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236(1):254–260. https://doi.org/10.1148/radiol.2361040450
Riederer I, Karampinos DC, Settles M, Preibisch C, Bauer JS, Kleine JF et al (2014) Double inversion recovery sequence of the cervical spinal cord in multiple sclerosis and related inflammatory diseases. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4093
Hodel J, Outteryck O, Bocher AL, Zephir H, Lambert O, Benadjaoud MA et al (2014) Comparison of 3D double inversion recovery and 2D STIR FLAIR MR sequences for the imaging of optic neuritis: pilot study. Eur Radiol. https://doi.org/10.1007/s00330-014-3342-3
Hadhoum N, Hodel J, Defoort-Dhellemmes S, Duhamel A, Drumez E, Zephir H et al (2015) Length of optic nerve double inversion recovery hypersignal is associated with retinal axonal loss. Mult Scler. https://doi.org/10.1177/1352458515598021
Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L et al (2016) MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 15(3):292–303. https://doi.org/10.1016/S1474-4422(15)00393-2
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Sartoretti T, Sartoretti E, Rauch S, Binkert C, Wyss M, Czell D et al (2017) How common is signal-intensity increase in optic nerve segments on 3D double inversion recovery sequences in visually asymptomatic patients with multiple sclerosis? AJNR Am J Neuroradiol 38(9):1748–1753. https://doi.org/10.3174/ajnr.A5262
Filippi M, Preziosa P, Meani A, Ciccarelli O, Mesaros S, Rovira A et al (2018) Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol 17(2):133–142. https://doi.org/10.1016/S1474-4422(17)30469-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the local ethics committee. Written informed consent of individual patients was waived for this retrospective analysis of anonymized data according to institutional guidelines.
Rights and permissions
About this article
Cite this article
Riederer, I., Mühlau, M., Hoshi, MM. et al. Detecting optic nerve lesions in clinically isolated syndrome and multiple sclerosis: double-inversion recovery magnetic resonance imaging in comparison with visually evoked potentials. J Neurol 266, 148–156 (2019). https://doi.org/10.1007/s00415-018-9114-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9114-2