Abstract
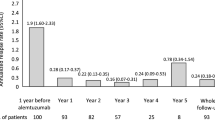
In this retrospective, multicenter, real-world study we collected clinical and magnetic resonance imaging (MRI) data of all patients (n = 40) with relapsing-remitting multiple sclerosis (RRMS) treated with alemtuzumab according to a “free-of-charge” protocol available before the drug marketing approval in Italy. Almost all (39/40) started alemtuzumab after discontinuing multiple disease-modifying treatments (DMTs) because of either lack of response or safety concerns. We considered the proportion of alemtuzumab-treated patients who had no evidence of disease activity (NEDA-3) and disability improvement over a 36-month follow-up period. NEDA-3 was defined as absence of relapses, disability worsening, and MRI activity. Disability improvement was defined as a sustained reduction of ≥ 1-point in Expanded Disability Status Scale (EDSS) score. At follow-up, 18 (45%) patients achieved NEDA-3, 30 (75%) were relapse-free, 33 (82.5%) were EDSS worsening-free, and 25 (62.5%) were MRI activity-free. Eleven (27.5%) patients had a sustained disability improvement. We found no predictor for the NEDA-3 status, while the interaction of higher EDSS score by higher number of pre-alemtuzumab relapses was associated with a greater chance of disability improvement (odds ratio 1.10, p = 0.049). Our study provides real-world evidence that alemtuzumab can promote clinical and MRI disease remission, as well as disability improvement, in a significant proportion of patients with RRMS despite prior multiple DMT failures. The drug safety profile was consistent with data available from clinical trials.



Similar content being viewed by others
References
CAMMS223 Trial Investigators, Coles AJ, Compston DAS et al (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359(17):1786–1801
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839
Coles AJ, Fox E, Vladic A et al (2012) Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 78(14):1069–1078
Havrdova E, Arnold DL, Cohen JA et al (2017) Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 89(11):1107–1116
Coles AJ, Cohen JA, Fox EJ et al (2017) Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 89(11):1117–1126
Arnold DL, Fisher E, Brinar VV et al (2016) Superior MRI outcomes with alemtuzumab compared with subcutaneous interferon β-1a in MS. Neurology 87(14):1464–1472
Giovannoni G, Cohen JA, Coles AJ et al (2016) Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology 87(19):1985–1992
Coles AJ, Cox A, Le Page E et al (2006) The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 253(1):98–108
Le Page E, Deburghgraeve V, Lester M-A et al (2015) Alemtuzumab as rescue therapy in a cohort of 16 aggressive multiple sclerosis patients previously treated by Mitoxantrone: an observational study. J Neurol 262(4):1024–1034
Tuohy O, Costelloe L, Hill-Cawthorne G et al (2015) Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 86(2):208–215
Willis MD, Harding KE, Pickersgill TP et al (2016) Alemtuzumab for multiple sclerosis: Long term follow-up in a multi-centre cohort. Mult Scler 22(9):1215–1223
Huhn K, Bayas A, Doerck S et al (2018) Alemtuzumab as rescue therapy in a cohort of 50 relapsing-remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265(7):1521–1527
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Filippi M, Rocca MA, Bastianello S et al (2013) Guidelines from The Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci 34(12):2085–2093
Giovannoni G, Turner B, Gnanapavan S et al (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4(4):329–333
Río J, Nos C, Tintoré M et al (2006) Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol 59(2):344–352
Phillips JT, Giovannoni G, Lublin FD et al (2011) Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 17(8):970–979
Altay EE, Fisher E, Jones SE et al (2013) Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol 70(3):338–344
ICH-GCP (2018) Available at: http://ichgcp.net/12-adverse-event-ae. Accessed 25 July 2018
Berger T, Elovaara I, Fredrikson S et al (2017) Alemtuzumab use in clinical practice: recommendations from European Multiple Sclerosis Experts. CNS Drugs 31(1):33–50
Hauser SL, Bar-Or A, Comi G et al (2017) Ocrelizumab versus Interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 376(3):221–234
Kalincik T, Brown JWL, Robertson N et al MSBase Study Group (2017) Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 16(4):271–281
Prosperini L, De Angelis F, De Angelis R, Fanelli F, Pozzilli C (2015) Sustained disability improvement is associated with T1 lesion volume shrinkage in natalizumab-treated patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 86(2):236–238
Jones JL, Anderson JM, Phuah C-L et al (2010) Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 133(Pt 8):2232–2247
Belachew S, Phan-Ba R, Bartholomé E et al (2011) Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing-remitting multiple sclerosis. Eur J Neurol 18(2):240–245
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ (2004) Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome. Health Technol Assess 8(9):1–48
Caon C, Namey M, Meyer C et al (2015) Prevention and management of infusion-associated reactions in the comparison of alemtuzumab and Rebif(®) Efficacy in multiple sclerosis (CARE-MS) program. Int J MS Care 17(4):191–198
Bianco A, Gambaro G, Rossini PM, Mirabella M (2015) Acyclovir-related kidney injury during alemtuzumab infusion. J Neurol 262(7):1772–1774
Moiola L, Pisa M, D’Angelo A, Martinelli V, Comi G (2017) First reported case of acquired Hemophilia A (AHA) as secondary autoimmune disease following alemtuzumab treatment in multiple sclerosis. Presented at the 7th joined ECTRIMS-ACTRIMS Meeting, Paris, France. Mult Scler 23(Suppl 3):P757
Martínez-Yélamos S, Martínez-Yélamos A, Martín Ozaeta G et al (2006) Regression to the mean in multiple sclerosis. Mult Scler 12(6):826–829
Stellmann JP, Sturner KH, Young KL et al (2015) Regression to the mean and predictors of MRI disease activity in RRMS placebo cohorts—is there a place for baseline-to-treatment studies in MS? PLoS One 10(2):e0116559
Acknowledgements
The Italian Alemtuzumab Study Group Angelo Ghezzi, Mauro Zaffaroni, Damiano Baroncini (S. Antonio Abate Hospital, Gallarate (VA), Italy); Fabio Buttari, Diego Centonze (Department of Neurosciences. Tor Vergata University, Rome, Italy); Arianna Fornasiero, Marco Salvetti (Department of Neurosciences, Mental Health and Sensory Organs, Sapienza University, S. Andrea Hospital, Rome, Italy); Renato Docimo, Elisabetta Signoriello, Gioacchino Tedeschi (Department of Medical, Surgical, Neurological, Metabolic and Aging Sciences, University of Campania “Luigi Vanvitelli”, Naples, Italy); Antonio Bertolotto, Marco Capobianco (Regional MS Centre, S. Luigi Gonzaga Hospital, Orbassano (TO), Italy); Giancarlo Comi (Department of Neurology, Institute of Experimental Neurology, Division of Neuroscience, S. Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy); Eleonora Cocco (MS Center, Binaghi Hospital, ASSL Cagliari, ATS regione Sardegna, Cagliari, Italy); Paolo Gallo, Marco Puthenparampil (Department of Neurosciences, MS Centre, University Hospital, Padova, Italy); Roberta Grasso, Valeria Di Francescantonio (Department Medical and Surgical Sciences, University of Foggia, Italy); Maria Rosaria Rottoli (USS Malattie Autoimmuni, A. O. Papa Giovanni XXIII, Bergamo, Italy); Massimiliano Mirabella (Fondazione Policlinico A. Gemelli, IRCCS, Rome, and Università Cattolica del Sacro Cuore, Roma, Italy); Alessandra Lugaresi, Giovanna De Luca, Maria Di Ioia, Valeria Di Tommaso, Luca Mancinelli (Department of Neuroscience and Imaging, University “G. d’Annunzio”, Chieti, Italy); Giancarlo Di Battista (Neurology Unit, S. Filippo Neri Hospital, Rome, Italy); Ada Francia, Serena Ruggieri, Carlo Pozzilli (Department of Neurology and Psychiatry, Sapienza University, Rome, Italy); Erica Curti, Elena Tsantes (Department of Medicine and Surgery, University of Parma, Parma, Italy); Barbara Palmeri (U.O.C. Neurologia, Fondazione Istituto “G.Giglio”, Cefalù (PA), Italy); Caterina Lapucci, Giovanni Luigi Mancardi, Antonio Uccelli (Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genova, Genova, Italy); Clara Chisari, Emanuele D’Amico (Department of Medical and Surgical Sciences and Advanced Technologies, Neuroscience Section, University of Catania, Italy); Elisabetta Cartechini (UOC Neurologia, ASUR Marche - AV3, Macerata, Italy); Anna Maria Repice (Neurologia II AOU Careggi Firenze, Firenze, Italy); Eliana Magnani, Luca Massaccesi (Department of Neurofarba University of Florence, Florence, Italy); Paolo Calabresi, Massimiliano Di Filippo, Maria Di Gregorio (Neurologic Clinic. Ospedale Santa Maria della Misericordia. University of Perugia, Perugia, Italy).
Funding
This study was funded by Sanofi Genzyme. The funder provided financial resources for the study conduction, but had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. LP: speaker honoraria from Almirall, Biogen, Genzyme, Novartis, Roche and Teva; consulting fees from Biogen, Genzyme and Novartis; research grant from Genzyme and Italian MS Society. PA: consulting and/or lecture fees from Mylan, Roche, Almirall e Merck, Biogen, Genzyme, Novartis and Teva. LB: lecture fees from Merck Serono and Teva. MCB: Merck Serono, Teva, Genzyme, Novartis (scientific board); Biogen, Teva (speaker). AG: speaker and consulting fees from Biogen, Sanofi-Genzyme, Merck Seronoand Teva. MM: honoraria from: Biogen, Novartis, Almirall, Teva. LM: speaking honoraria and/or consultant fees from Biogen, Merck Serono, Sanofi-Aventis, Teva, Novartis. LM: speaking honoraria and/or consultant fees from Biogen, Genzyme and Teva. PP: speaking honoraria and/or consultant fees from Biogen, Merck serono, Teva, Novartis and Genzyme. CA: speaking honoraria and/or consultant fees from Merck Serono, Novartis, Genzyme. VB: speaking honoraria and/or consultant fees from Biogen Idec, Merck Serono, Bayer, Sanofi- Genzyme, Novartis. AB: nothing to disclose. DF: scientific advisory board and speaker honoraria from Merck Serono, Biogen, Sanofi-Genzyme, Bayer Shering and Novartis. EF: nothing to disclose. AF: research funding and lecture fees from Sanofi-Aventis, Biogen Idec, Merck Serono and Novartis. FG: research funding from Sanofi-Aventis and Biogen; advisory boards and speaking honoroaria from Biogen, Novartis, Sanofi Aventis, Merck Serono and Roche. LMEG: scientific advisory board for Merck Serono; funding for travel or speaker honoraria from Merck Serono, Biogen, Sanofi-Aventis, Bayer Schering and Solvay Pharmaceuticals, Inc.; institutional research support form Teva Pharmaceuticals Industries Ltd, Biogen, Genzyme Corporation, Sanofi-Aventis, Merck Serono, Novartis and Eisai Inc.; research support from Merck Serono, Biogen and Ministero della Salute of Italy. AL: lecturing honoraria from Biogen, Novartis and Teva; consulting fees from Sanofi-Genzyme, Biogen, Merck, Roche; funding for travel from Sanofi-Genzyme, Biogen, Merck Serono, and Teva. GL: scientific advisory boards for Almirall, Novartis, Biogen Idec, Sanofi-Aventis, Genzyme and Bayer Schering; funding for travel and speaker honoraria from Sanofi-Aventis, Biogen Idec, Bayer Schering, Teva Neurosciences, Almirall, Genzyme and Novartis; research support from Novartis, ‘Fondazione C. Serono’, Biogen Idec, Bayer Schering and Sanofi-Aventis. FP: scientific advisory board for Almirall, Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme, Roche and TEVA; fees for speaking activities by Almirall, Bayer, Biogen, Merck, Novartis, Sanofi-Genzyme and TEVA. EP: personal fees and non-financial support from Biogen Idec, Merck Serono, Teva, Genzyme and Novartis; non-financial support from “Associazione Marchigiana Sclerosi Multipla e Altre Malattie Neurologiche”. MP: nothing to disclose. PS: nothing to disclose.
Ethical standards
The present study was conducted after approval of the Ethics Committee of Sapienza University (application no. 2923/15 - RIF.CE: 3947 of 17 December 2015). All data were gathered after informed consent was obtained from each participant, in accordance with specific national laws and the ethics standards laid down in the 1964 Declaration of Helsinki and its later amendments. In no way this study did interfere in the care received by patients. Anonymized data will be shared upon request by the Principal Investigator (LP).
Additional information
The members of the the Italian Alemtuzumab Study Group are listed in the Acknowledgements and in the electronic supplementary material.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prosperini, L., Annovazzi, P., Boffa, L. et al. No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol 265, 2851–2860 (2018). https://doi.org/10.1007/s00415-018-9070-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9070-x