Abstract
Background
Dimethyl-fumarate (DMF) demonstrated efficacy and safety in relapsing–remitting multiple sclerosis (MS) in randomized clinical trials.
Objectives
To track and evaluate post-market DMF profile in real-world setting.
Materials and methods
Patients receiving DMF referred to Italian MS centres were enrolled and prospectively followed, collecting demographic clinical and radiological data.
Results
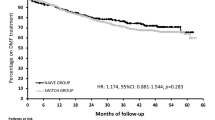
Among the 735 included patients, 45.4% were naïve to disease-modifying therapies, 17.8% switched to DMF because of tolerance, 27.4% switched to DMF because of lack of efficacy, and 9.4% switched to DMF because of safety concerns. Median DMF exposure was 17 months (0–33). DMF reduced the annual relapse rate (ARR) by 63.2%. At 12 and 24 months, 85 and 76% of patients were relapse-free. NEDA-3 status after 12 months of DMF treatment was maintained by 47.5% of patients. 89 and 70% of patients at 12 and 24 months regularly continued DMF. Most frequent adverse events (AEs) were flushing (37.2%) and gastro-enteric AEs (31.1%).
Conclusion
Our post-market study corroborated that DMF is a safe and effective drug. Additionally, the study suggested that naïve patients strongly benefit from DMF and that DMF improved ARR also in patients who were horizontally switched from injectable therapies due to tolerability and efficacy issues.




Similar content being viewed by others
References
Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Investigators CS (2012) Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT, Investigators DS (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367:1098–1107
Gold R, Arnold DL, Bar-Or A, Hutchinson M, Kappos L, Havrdova E, MacManus DG, Yousry TA, Pozzilli C, Selmaj K, Sweetser MT, Zhang R, Yang M, Potts J, Novas M, Miller DH, Kurukulasuriya NC, Fox RJ, Phillips TJ (2017) Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: interim analysis of ENDORSE, a randomized extension study. Mult Scler 23:253–265
Trojano M, Tintore M, Montalban X, Hillert J, Kalincik T, Iaffaldano P, Spelman T, Sormani MP, Butzkueven H (2017) Treatment decisions in multiple sclerosis—insights from real-world observational studies. Nat Rev Neurol 13:105–118
Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, Achiti I, Confavreux C, Coustans M, le Page E, Edan G, McDonnell GV, Hawkins S, Trojano M, Liguori M, Cocco E, Marrosu MG, Tesser F, Leone MA, Weber A, Zipp F, Miterski B, Epplen JT, Oturai A, Sorensen PS, Celius EG, Lara NT, Montalban X, Villoslada P, Silva AM, Marta M, Leite I, Dubois B, Rubio J, Butzkueven H, Kilpatrick T, Mycko MP, Selmaj KW, Rio ME, Sa M, Salemi G, Savettieri G, Hillert J, Compston DA (2005) Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology 64:1144–1151
Giovannoni G, Tomic D, Bright JR, Havrdova E (2017) “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 23:1179–1187
Gallo P, Van Wijmeersch B, Paradig MSG (2015) Overview of the management of relapsing-remitting multiple sclerosis and practical recommendations. Eur J Neurol 22(Suppl 2):14–21
Kresa-Reahl K (2016) Clinical measures and impact on patient-reported outcomes of delayed-release dimethyl fumarate in relapsing multiple sclerosis patients after suboptimal response to glatiramer acetate: analysis of the 12-month RESPOND study. ECTRIMS, ECTRIMS Online Library, London, UK
Miclea A, Leussink VI, Hartung HP, Gold R, Hoepner R (2016) Safety and efficacy of dimethyl fumarate in multiple sclerosis: a multi-center observational study. J Neurol 263:1626–1632
Fernandez O, Giovannoni G, Fox RJ, Gold R, Phillips JT, Potts J, Okwuokenye M, Marantz JL (2017) Efficacy and safety of delayed-release dimethyl fumarate for relapsing-remitting multiple sclerosis in prior interferon users: an integrated analysis of DEFINE and CONFIRM. Clin Ther 39:1671–1679
Havrdova E, Giovannoni G, Gold R, Fox RJ, Kappos L, Phillips JT, Okwuokenye M, Marantz JL (2017) Effect of delayed-release dimethyl fumarate on no evidence of disease activity in relapsing-remitting multiple sclerosis: integrated analysis of the phase III DEFINE and CONFIRM studies. Eur J Neurol 24:726–733
Berglund A (2016) Comparative analysis of adherence and persistence for delayed-release dimethyl fumarate versus interferons and glatiramer acetate—a population based study in Sweden. In: ECTRIMS. ECTRIMS Online Library, London, UK
Alroughani R, Ahmed SF, Behbehani R, Al-Hashel J (2017) Effectiveness and safety of dimethyl fumarate treatment in relapsing multiple sclerosis patients: real-world evidence. Neurol Ther 6:189–196
Fox EJ, Vasquez A, Grainger W, Ma TS, von Hehn C, Walsh J, Li J, Zambrano J (2016) Gastrointestinal tolerability of delayed-release dimethyl fumarate in a multicenter, open-label study of patients with relapsing forms of multiple sclerosis (MANAGE). Int J MS Care 18:9–18
Phillips JT, Hutchinson M, Fox R, Gold R, Havrdova E (2014) Managing flushing and gastrointestinal events associated with delayed-release dimethyl fumarate: experiences of an international panel. Mult Scler Relat Disord 3:513–519
Theodore Phillips J, Erwin AA, Agrella S, Kremenchutzky M, Kramer JF, Darkes MJ, Kendter J, Abourjaily H, Rana J, Fox RJ (2015) Consensus management of gastrointestinal events associated with delayed-release dimethyl fumarate: a Delphi study. Neurol Ther 4:137–146
Longbrake EE, Naismith RT, Parks BJ, Wu GF, Cross AH (2015) Dimethyl fumarate-associated lymphopenia: risk factors and clinical significance. Mult Scler J Exp Transl Clin 1:2055217315596994. https://doi.org/10.1177/2055217315596994
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
G. Mallucci received support to travel to scientific meetings from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, and Teva; received speaker honoraria from Biogen Idec and served on the scientific advisory board for Genzyme and Merck Serono. P. Annovazzi received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Merck, Biogen, Teva, Sanofi-Genzyme, Almirall, Mylan, Roche and Novartis. S.Miante received a travel grant form Biogen Idec, Novartis, Sanofi-Aventis and Teva. V. Torri-Clerici acted as an Advisory Board member of Merck Serono, Novartis and Genzyme, and received funding for travelling and honoraria for speaking or writing or consultancy from Biogen Idec, Merck, Teva, Novartis, Genzyme, and Almirall. She received support for research project by Almirall and is involved as principal investigator in clinical trials for Novartis and Merck. M. Matta has nothing to disclose. S. La Gioia received grants from Biogen for travel and congress participation. R. Cavarretta has nothing to disclose. V. Mantero has nothing to disclose. G. Costantini has nothing to disclose. V. D’Ambrosio has nothing to disclose. M. Zaffaroni received honoraria for consultancy and participation in advisory boards or travel grants from Genzyme, Biogen Idec, Merck Serono, Sanofi-Aventis, Teva, and Novartis. A. Ghezzi has served on scientific advisory boards for Merck Serono, Biogen Idec and Teva Pharmaceutical Industries Ltd; has received speaker honoraria from Merck Serono, Biogen Idec, Bayer Schering Pharma Novartis, and Serono Symposia International; served as a consultant for Novartis; and receives research support from Sanofi-Aventis, Biogen Idec and Merck Serono. P. Perini has received funding for travel and speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis, and Bayer Schering Pharma and has been a consultant for Merck Serono, Biogen Idec and Teva. S. Rossi acted as an Advisory Board member of Biogen Idec, Bayer Schering, Merck Serono, Teva, Novartis and Genzyme, and received funding for travelling and honoraria for speaking or writing from Biogen Idec, Merck Serono, Teva, Novartis, Bayer Schering, Genzyme, Almirall. She received support for research project by Teva, Merck Serono and Bayer Schering and is involved as principal investigator in clinical trials for Teva and Roche. A. Bertolotto served on the scientific advisory boards of Almirall, Bayer, BiogenIdec, and Genzyme; received speaker honoraria from BiogenIdec, Genzyme, Novartis, and Teva; his institution has received grant support from Bayer, BiogenIdec, Merck, Novartis, Teva, the Italian Multiple Sclerosis Society, Fondazione Ricerca Biomedica ONLUS, and San Luigi ONLUS; received speaker honoraria from BiogenIdec, Genzyme, Novartis, Sanofi-Aventis, and Teva; is on the editorial board of Multiple Sclerosis International, Progress in Neuroscience, Dataset Papers in Neuroscience, Journal of Multiple Sclerosis, Neurology and Therapy, and Multiple Sclerosis and Demyelinating Disorders; and received research support from Regione Piemonte, Italian Multiple Sclerosis Society, Associazione Ricerca Biomedica ONLUS, and San Luigi ONLUS. MR Rottoli has nothing to disclose. M. Rovaris received compensation from Biogen Italy, Teva Pharmaceuticals, Novartis, and Genzyme for speaking and consultant fees. Dr. Rovaris received financial support for research activities from Teva Pharmaceuticals and Merck Serono. R. Balgera has nothing to disclose. P. Cavalla acted as an Advisory Board member of Merck Serono and Genzyme, and received funding for travelling and honoraria for speaking or consultancy from Biogen Idec, Merck Serono, Teva, Novartis, Genzyme and Almirall. She is involved as principal investigator in clinical trials for Novartis, Biogen and Genzyme. C. Montomoli received teaching grants from Biogen Idec and Merck Serono. R. Bergamaschi received research grants from Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva received lecture honoraria from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis and Teva; and received support to travel to scientific meetings from Almirall, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis and Teva.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mallucci, G., Annovazzi, P., Miante, S. et al. Two-year real-life efficacy, tolerability and safety of dimethyl fumarate in an Italian multicentre study. J Neurol 265, 1850–1859 (2018). https://doi.org/10.1007/s00415-018-8916-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8916-6