Abstract
Lewy body dementia (DLB) is a common form of cognitive impairment, accounting for 30% of dementia cases in ages over 65 years. Early diagnosis of DLB has been challenging; particularly in the context of differentiation with Parkinson’s disease dementia and other forms of dementias, such as Alzheimer’s disease and rapidly progressive dementias. Current practice involves the use of [123I]FP-CIT-SPECT, [18F]FDG PET and [123I]MIBG molecular imaging to support diagnostic procedures. Structural imaging techniques have an essential role for excluding structural causes, which could lead to a DLB-like phenotype, as well as aiding differential diagnosis through illustrating disease-specific patterns of atrophy. Novel PET molecular imaging modalities, such as amyloid and tau imaging, may provide further insights into DLB pathophysiology and may aid in early diagnosis. A multimodal approach, through combining various established techniques and possibly using novel radioligands, might further aid towards an in-depth understanding of this highly disabling disease. In this review, we will provide an overview of neuroimaging applications in patients with DLB.
Similar content being viewed by others
Introduction
Dementia with Lewy bodies (DLB) is a common form of cognitive impairment, accounting for substantial clinical deterioration and a significant burden in patients and caregivers [1]. The classic presentation of DLB encompasses tandem features of fluctuating cognitive decline, parkinsonism and visual hallucinations [1]. Conjointly with Parkinson’s disease dementia (PDD), they comprise a spectrum of neurodegenerative dementias that share the common hallmark of α-synuclein pathology [2]. Thus, the term Lewy body disease is currently used to describe neurodegenerative conditions with similar clinical phenotype (dementia combined with parkinsonism) and underlying pathophysiology [3]. Aggregation of α-synuclein (SNCA) in Lewy bodies and neurites often coexists with amyloid-β plaques and tau neurofibrillary tangles [4]. An integrated approach in these conditions that have a consecutive clinical outcome is ideal for elucidating underlying mechanisms and therefore improving diagnostic tools and therapeutic interventions.
Diagnostic criteria for DLB harbor an acceptable sensitivity [1]. However, specificity and diagnostic accuracy in the clinical setting remain as challenges to be further addressed. In the clinical setting, DLB is often misdiagnosed [5]. Consequently, patients are prone to non-beneficial or even harmful treatment options and incomplete disease management [6]. Clinically relevant biomarkers could potentially contribute to an enhanced diagnostic accuracy [7]. Detecting lower levels of α-synuclein in the CSF of patients with suspected DLB has been proven of potential utility, especially in discriminating from Alzheimer’s disease (AD) [8]. Alas, reliability of CSF or serum biomarkers to serve as positive diagnostic tools is not yet consistent.
In respect of the former and considering that DLB diagnosis relies predominately on clinical features, neuroimaging biomarkers could aid towards an increased diagnostic certainty [9]. Besides excluding secondary causes of dementia using structural imaging, neuroimaging modalities can also be implemented in aiding differential diagnosis and investigating underlying pathophysiological mechanisms (Table 1). However, the application of advanced techniques in the clinical setting requires additional validation. In this review, we will provide an overview of neuroimaging modalities currently used to assess patients with DLB.
Diagnosis of DLB
Diagnosis of DLB continues to heavily rely on clinical manifestations of the disease, as structural neuroimaging lacks definitive characteristics with significant diagnostic value [10]. In DLB, cognitive decline either antedates or occurs simultaneously with parkinsonism, whereas in PDD it follows the constellation of parkinsonism. Key characteristics of DLB, which are less common in PDD, include fluctuating cognition and sensitivity to neuroleptics [6]. Supportive features in DLB diagnosis includes relatively preserved medial temporal lobe structures as seen on CT or MRI [10]. Though this feature is commonly present, it has not been proven to have adequate diagnostic specificity. Current diagnostic criteria have included the use of [123I]FP-CIT-SPECT, [18F]FDG PET and [123I]MIBG as supportive or indicative diagnostic features. Recently, besides imaging biomarkers, other clinical measures (polysomnography, electroencephalography) have been incorporated in the diagnostic criteria [9].
Structural imaging
Structural brain changes can be visualized and assessed using MRI and CT, providing a measure of cerebral atrophy, as well as white matter integrity in DLB. Structural neuroimaging is often utilized in the clinical setting for differential diagnosis of various types of dementia [11]. These imaging techniques are primarily used to detect cerebrovascular diseases and space-occupying lesions such as brain tumor or hematoma [12, 13]. Though CT is most often used clinically due to its relatively low cost and widespread availability, MRI offers superior contrast, as well as specific tissue characterisation (Table 2). An array of analyses have been developed and performed, including whole brain analyses (voxel-based morphometry, cortical thickness), region of interest (ROI) analyses and visual inspection, to compare regional structural changes in patients with DLB to those with Alzheimer’s disease, Parkinson’s disease dementia and healthy controls.
Comparison between DLB and AD
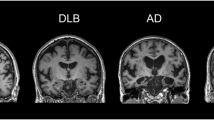
MRI has been widely used to investigate patterns of gray matter (GM) atrophy. Advances in image processing have enabled automatic extraction of whole brain cortical thickness, which is retrieved from structural MRI. Although DLB has demonstrated some overlap with the cortical atrophy patterns seen in AD, atrophy is generally less diffuse in DLB with moderate preservation of the medial temporal lobe structures [14,15,16].
Cortical thickness assessment has also been shown to have high precision and sensitivity in identifying morphological changes, which arise from neuropathological changes. This method has, therefore, been employed in several studies as a way to differentiate DLB from AD, PDD and healthy controls. Investigations into cortical thickness alterations in DLB revealed relatively small GM change, primarily affecting the posterior parietal areas, as opposed to the patterns of GM change affecting the temporoparietal association cortices in AD [17]. These findings are in corroboration with the notion that DLB is a result of neuronal synaptic dysfunction, not neuronal loss. Through carrying out a multivariate classification study of cortical thickness, Lebedev and colleagues demonstrated that this method has the ability to differentiate DLB from AD with 82.1% sensitivity and 85.7% specificity [18]. Specifically, AD was characterized by patterns of cortical thinning within the temporal pole, subgenual cingulate regions and the parahippocampus, whereas regional thinning was localized to the superior temporo-occipital and lateral orbito-frontal regions, as well as the middle and posterior cingulate in DLB [18]. The finding of AD exhibiting greater temporal involvement compared to DLB has been a homogenous result across several structural imaging studies [19, 20].
Although investigations into hippocampal atrophy have revealed that DLB patients have less severe atrophy compared to AD patients [21, 22], with the entorhinal cortex, CA1 and subiculum areas of the hippocampus being most affected in AD [23], recently, Delli Pizzi et al. explored the differential contribution of hippocampal subfields and adjacent extrahippocampal structures to the pathophysiology of AD and DLB [24]. They reported that the cornu ammonis and subiculum were preserved in DLB, but the perirhinal cortex and parahippocampus were damaged, highlighting the differential alteration of hippocampal subfields and adjacent extrahippocampal structures in DLB and AD [24].
Studies have also demonstrated greater atrophy in the substantia innominate, with increased dorsal mesopontine GM atrophy distinguishing patients with clinically diagnosed DLB from AD [25]. These findings may suggest a greater cholinergic dysfunction in DLB, perhaps related to the presence of midbrain synuclein pathology.
Using diffusion tensor MRI, Watson et al. revealed that the parieto-occipital white matter tracts were preferentially affected in DLB, though this appears to be an early phenomenon, as AD demonstrated a greater longitudinal increase in mean diffusivity in parietal and temporal regions compared to DLB, with no evidence of longitudinal changes in mean diffusivity or fractional anisotropy in DLB relative to controls [26]. However, DLB was differentiable from AD given that it was associated with reduced fractional anisotropy in the pons and left thalamus, highlighting that, despite similar levels of dementia severity, patterns of DTI changes in DLB and AD varied [26].
A recent study by Shams et al. demonstrated that MRI of the swallow tail sign may have diagnostic potential in DLB [27], given that the largest dopamine-containing cluster within caudal and posterolateral part of the substantia nigra (nigrosome 1) is highly affected in parkinsonian syndromes. More specifically, Shams et al. reported that a hypointense nigrosome 1, as visualized on iron-sensitive susceptibility-weighted imaging (SWI), was more common in DLB compared to AD, frontotemporal dementia and controls. This was in corroboration with Kamagata et al. who reported that measuring nigrosome 1 hypointensity with SWI achieved 90% diagnostic accuracy (93% sensitivity and 87% specificity) in DLB [28].
Comparison between DLB and PDD
Attempts to compare GM loss between DLB and PDD have revealed a pattern of more pronounced GM loss in DLB compared to PDD, which is in line with the fact that DLB encompasses greater amyloid burden [29]. It is important to note, however, that localisations of GM reductions in DLB relative to PDD vary amongst different studies. For example, Burton et al. were unable to identify distinct cortical atrophy profiles of DLB and PDD [30], but Beyer et al. reported GM reductions in the temporal, parietal and occipital lobes in DLB using a voxel-based morphometry (VBM) approach [31]. Alongside the temporal and parietal atrophy, Lee et al. also reported occipital and striatal GM reductions in DLB [32]. Studies investigating correlation patterns between brain structure and clinical and neuropsychiatric manifestations of the disease, revealed that decreased GM volume of the anterior cingulate, right hippocampus and amygdala were associated with cognitive performance [33], whilst reduced GM volume in the left precuneus and inferior frontal lobe correlated with visual hallucinations in DLB, but not in PDD [34].
Functional imaging
Active task and resting state functional MRI (fMRI) are the primary tools employed to investigate cerebral function associated to cognitive tasks or during rest, respectively, by measuring changes in blood-oxygen level-dependent (BOLD) signal.
Comparison between DLB and AD
Although only a few fMRI studies have examined BOLD signal in DLB, differential patterns of functional connectivity in DLB compared to AD have been reported (Table 2). Using the precuneus as the seed region, Galvin et al. reported that DLB patients exhibit increased connectivity in the inferior parietal cortex and putamen, and decreased connectivity in the fronto-parietal operculum, medial prefrontal cortex and the primary visual cortex compared to AD, whilst a reversal of connectivity was observed in the right hippocampus [35]. Independent component analysis (ICA) has demonstrated that DLB display greater connectivity in the default mode network compared to AD [36], which contrasts with previously reported connectivity dysfunctions between anterior and posterior segments of the default mode network in AD, when compared to healthy controls [37]. Furthermore, increased connectivity between the putamen and frontal, temporal and parietal regions has been illustrated by DLB patients in comparison to AD patients, with the authors suggesting that this may be related to the prominent parkinsonian features in DLB [38]. Consistent with the moderate preservation of memory function observed in DLB as opposed to AD, hippocampal connectivity has not been shown to differ in DLB compared to healthy controls, though the left hippocampal connectivity was identified to be higher in AD compared to controls, potentially reflecting a compensatory mechanism [38].
A recent study by Schumacher et al. aimed to explore within- and between-network connectivity in a range of resting state networks, being the first to investigate how DLB affects connectivity between these resting state networks [39]. DLB patients displayed more decreases in within-network connectivity compared to controls, primarily in temporal, motor and frontal networks. In contrast, long-range functional connectivity appeared to be intact in DLB, with increased connectivity only identified between a frontal and a temporal network [39]. Only subtle differences were observed when AD and DLB were compared, suggesting a potential overlap in their resting state functional connectivity.
Given the prominent prevalence of visuoperceptual impairments in DLB, a task-based fMRI study employed visual presentations of motion, color and face paradigms to explore the functional integrity of the visual system in DLB. They discovered that DLB patients exhibited greater activation in the superior temporal sulcus compared to AD, specifically during the motion task [40]. However, these findings were not replicated by Taylor et al., who reported that DLB patients did not exhibit any significant differences in functional response to objects, motion stimuli or checkerboard in V1 and V2/V3 compared to controls [41], proposing that function in the lower visual areas is relatively preserved. Interestingly, however, ROI analysis demonstrated that the DLB group had a reduction in V5/MT (middle temporal) activation when responding to motion stimuli [41]. Taken together, these results imply that, in DLB, functional abnormalities affect the visual association areas, as opposed to the primary visual cortex, though it is difficult to decipher whether deviations at higher levels of the visual system contribute to the hallmark visuoperceptual impairments and visual hallucinations seen in DLB.
Comparison between DLB and PDD
Although studies have demonstrated alterations in functional connectivity in PDD [42,43,44,45] and DLB [35, 38, 46,47,48], these were reported when comparing these disease groups against healthy controls. One study has, however, compared DLB and PDD directly with the aim of identifying disease-specific functional connectivity patterns (Table 2). Peraza et al. reported that, for seeds situated within the fronto-parietal network, DLB patients exhibited greater alterations in functional connectivity than PDD when compared to healthy controls, predominately at the precentral and postcentral gyri, cerebellar, occipital and temporal regions, whilst in PDD, changes in functional connectivity were limited to the frontal cortices and precuneal [49]. Interestingly, although the supplementary motor area seed revealed similar regional functional connectivity alterations in the pre- and postcentral gyri, cerebellar, temporal, precuneal and occipital regions, these alterations were more apparent in PDD than in DLB, potentially reflecting the prominent parkinsonism and motor dysfunction in PDD compared to DLB [49]. However, Peraza et al. reported that no significant differences were found when DLB and PDD groups were compared to each other. Taken together, these results suggest that there are subtle functional differences between both diseases, which may be driven by their distinct pathological trajectories, thus potentially reflecting the chronological manifestation of cardinal symptoms in the Lewy body dementias.
Cortical and subcortical involvement in DLB
Serial MRI is an appealing biomarker of neurodegeneration and can assist in monitoring disease progression. Although longitudinal cerebral atrophy rates in AD are well-established and employed as outcome measures in clinical trials of potential disease-modifying agents, the atrophy rate in DLB has been reported to be analogous to or marginally greater than healthy controls [50]. While longitudinal studies of DLB are challenging given the higher mortality rates compared to AD [51], further investigations into DLB patients with a more rapidly progressive disease would be valuable in elucidating the neurobiological underpinnings of disease heterogeneity in DLB.
Evidence of subcortical involvement in DLB has revealed the vulnerability of the thalamus, striatum and brainstem to Lewy-related pathology. Studies have demonstrated that thalamic diffusion and perfusion deficits are associated with DLB [52], and striatal volumetric loss appears to be more affected in DLB than AD [53], with prominent nigrostriatal dysfunction [54]. Significant reductions in brainstem volume in DLB have also been reported [53], with Seidel et al. showing marked to severe neuronal loss in the ventral tegmental, pedunculopontine nucleus and locus coeruleus regions in DLB [54].
Cerebrovascular pathology
Although cerebrovascular pathology is common in older people, the contribution of vascular lesions to dementia remains to be elucidated. White matter damage can be visualized as focal punctate areas of high intensity signal using T2-weighted MRI. White matter hyperintensities (WMH) burden has been reported to be similar in DLB and AD [55], with DLB displaying no longitudinal change overtime relative to controls and baseline WMH burden predicting progression [56]. Interestingly, a study carried out by De Reuck et al. using a 7-Tesla scanner revealed that DLB patients had more cerebral microinfarcts compared to controls, with a higher abundance of the smallest lesions than vascular dementia and AD [57].
Cerebral microbleeds can be visualized using gradient-echo T2*-weighted MRI. A higher number of microbleeds has been reported in DLB than in AD, aside from the occipital lobes in one study [58]. DLB subjects with microbleeds have less abnormal MIBG scans, indicating that there is an inverse association between vascular lesions and Lewy body pathology [58]. Although Ballard et al. revealed that WMH in the basal ganglia and deep white matter appear to be associated with orthostatic hypotension in DLB [59], more work is required to evaluate the influence of vascular pathologies to the dementia syndrome, clinical features of DLB and its rate of progression.
Sensitivity and specificity of structural imaging modalities in pathologically proven DLB cases
Though scarce, studies have investigated the diagnostic accuracy of MRI for discriminating DLB from other dementias in autopsy-confirmed cases (Table 4). Both longitudinal and cross-sectional studies have illustrated that DLB is associated with less conspicuous global atrophy, compared to AD, with relative preservation of the medial temporal lobe [22]. Burton et al. aimed to determine the clinical relevance of visually rating the medial temporal lobe on MRI, and whether this technique could serve as an accurate diagnostic tool to distinguish AD from DLB and vascular cognitive impairment (VCI) [16]. In pathologically confirmed cases, medial temporal lobe atrophy served as a highly accurate diagnostic marker, with a sensitivity of 91% and specificity of 94%, in AD compared with DLB and VCI [16]. Medial temporal lobe atrophy scores did not differ between DLB and VCI. These results highlight that medial temporal lobe atrophy on MRI has robust discriminatory power for distinguishing AD from DLB. Furthermore, Burton et al. reported that medial temporal lobe atrophy is pathologically more strongly associated with neurofibrillary tangles and β-amyloid plaques, as opposed to Lewy body-like neuronal inclusions. These results are suggestive of gray matter atrophy, in DLB, arising as a result of concomitant AD-specific pathology. On the contrary, another postmortem MRI study assessing medial temporal lobe atrophy reported that this technique lacked discriminative potential, possessing an inability to exclude DLB diagnosis, particularly amongst patients who were over 85 years of age [60]. Although a strong relationship was found between medial temporal lobe atrophy and Alzheimer’s disease pathology, the sensitivity and specificity were 63 and 69%, respectively, for AD. Medial temporal lobe atrophy was also identified in subjects presenting with alternative primary hippocampal pathology, including Lewy-related pathology, highlighting the lack of specificity for AD-type pathology [60].
Recently, Harper et al. employed structural MRI and 184 post-mortem confirmed dementia cases to evaluate the reliability of six visual rating scales, including the medial temporal lobe atrophy scale, posterior atrophy scale, the anterior temporal scale, orbito-frontal, fronto-insula and anterior cingulate [61]. Using automated classification based on all six visual rating scales, the authors were able to distinguish pathological groups with an accuracy ranging from 86–97% from healthy controls, with DLB being distinguishable with sensitivity of 64% and specificity of 92%, leading to a balanced accuracy of 78% (Table 4). DLB was also differentiated from AD with a sensitivity of 64%, specificity of 82% and balanced accuracy of 73%, as well as from frontotemporal lobar degeneration (FTLD) with a sensitivity of 93%, specificity of 89% and balanced accuracy of 91%. The low sensitivity in distinguishing DLB from controls or AD ultimately emphasizes the elevated number of false negatives attached to DLB diagnosis, which is likely due to the large degree of overlap which exists between DLB and AD, as demonstrated by the fact that ~ 50% of DLB cases exhibit significant amyloid burden [62]. This was also demonstrated by Nedelska et al., who, in histopathologically confirmed cases, demonstrated that mixed DLB/AD cases exhibited markedly higher rates of brain atrophy, with the topography of changes corroborating with that seen in AD, predominantly affecting temporoparietal cortices, amygdala and hippocampi [63]. However, DLB patients exhibited minimal global atrophy compared to controls, with no region-specific atrophy that enabled distinguishability from controls [63]. The issue of false negative diagnoses has critical treatment implications, as failure to properly diagnose DLB clinically will likely result in limited use of existing symptomatic treatments, as well as exposure to non-beneficial or even harmful treatment options.
Molecular imaging
Molecular imaging has provided further insights into the pathophysiology of a complex disease such as DLB. Modalities such as single photon emission tomography (SPECT) and positron emission imaging (PET) are valuable methods of assessing neurobiology in vivo. Radionucleosides tracing neurotransmitters, synaptic pathology and misfolded protein aggregation provide elusive tools in investigating underlying disease mechanisms (Table 3).
Metabolic imaging
[18F]FDG PET is used in detecting cerebral glucose metabolism, which is impaired in cases of neuronal degeneration and synaptic pathology. It has been widely used in assessing dementias, and has been proven to be an effective tool in aiding the diagnosis of AD and monitoring its progression [64,65,66].
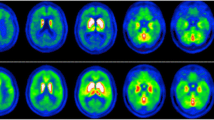
In DLB, the topographical pattern of hypometabolism includes mainly the occipital areas, visual association cortices and the posterior parietotemporal areas [67,68,69], though in AD, decreased cerebral metabolism tends to involve other areas as well [70]. In a recent multimodal PET study assessing amyloid-β deposition and cerebral glucose metabolism, with [11C]PiB and [18F]FDG, respectively, Chinese patients with probable DLB exhibited cortical amyloid-β deposition, as well as hypometabolism in the temporo-parieto-occipital region, insular, precuneus, frontal lobe, posterior cingulate and caudate nuclei [71].
Another characteristic feature of DLB is preserved metabolism in the posterior cingulate area when compared to the precuneus and cuneus [72]. This is called the cingulate island sign and can be related to the common visual hallucinations in patients with DLB. Furthermore, it harbors a notable sensitivity and specificity [66, 73]. The cingulate island sign has been inversely correlated with neurofibrillary tangle pathology in autopsy studies [73]. A recent study has also reported association of cingulate island sign, not only with medial temporal lobe atrophy, but with clinical symptoms (cognitive impairment, visual hallucinations) of DLB patients as well [73].
Imaging dopaminergic dysfunction
Dopamine transporter (DAT) imaging with SPECT using as a radiotracer [123I]FP-CIT-SPECT has been a valuable tool in assessing dopaminergic function in vivo. Decreased DAT uptake in basal ganglia is considered a supportive diagnostic feature according to current consensus diagnostic criteria [74, 75]. The diagnostic accuracy is even higher when applied in autopsy-proven cases of DLB [76,77,78]. Yielding a sensitivity of 88% and a specificity of 100% over non-DLB cases, [123I]FP-CIT-SPECT is a highly useful diagnostic tool [74]. A meta-analysis referring to 419 patients enrolled in 4 studies, showed a remarkable diagnostic accuracy, with a mean sensitivity of 86.5% and a mean specificity of 93.6% [79]. When comparing pathologically proven cases to clinical diagnosis, [123I]FP-CIT-SPECT has demonstrated increased accuracy in differentiating DLB from AD [80, 81]. In DLB, there is a decreased level of DAT, which is helpful in differentiating from AD where DAT is preserved [82, 83]. On the other hand, DAT imaging is not useful in discriminating DLB from PD-MCI and PDD, where there is a profound loss of DAT in the striatum [84]. Although DAT imaging possesses an inability to distinguish between parkinsonian syndromes, a recent study by Takaya et al. revealed that a combination of disease-specific perfusion patterns and striatal DAT activity accurately differentiates between atypical parkinsonian syndromes and Lewy body dementia [85]. However, in the rare cases of DLB where nigrostriatal degeneration is minimal and cortical pathology is the prominent feature, false negative results might occur. As for discriminating DLB patients from frontotemporal dementia or atypical parkinsonian syndromes (i.e. progressive supranuclear palsy (PSP), corticobasal degeneration (CBD)), [123I]FP-CIT-SPECT should not solely be accounted for as a reliable method of investigation [86]. The clinical phenotype should always be considered when interpreting findings regarding the above-mentioned conditions.
Imaging cardiac sympathetic innervation
[123I]MIBG cardiac scintigraphy is widely used to assess cardiac postganglionic sympathetic degeneration, which is a common feature in neurodegenerative diseases with Lewy Bodies pathology. [123I]MIBG is a promising biomarker with the ability of excluding AD and predicting conversion of possible to probable DLB [87,88,89]. A large multicenter study including 133 patients, diagnosed according to the consensus criteria, highlighted similar sensitivity and specificity to [123I]FP-CIT-SPECT [90, 91]. Although the [123I]MIBG is a credible modality, certain pitfalls should be considered. The presence of diabetes mellitus or cardiac disease might provide false positive results [92]. Thus, such patients should be excluded from undergoing cardiac scintigraphy for diagnostic purposes. When comorbidities are taken into account, [123I]FP-CIT-SPECT may have a distinguishable diagnostic significance in the clinical setting [93].
Amyloid imaging
Positive amyloid imaging is a classic feature of AD, with plaque deposition becoming apparent years after clinical symptomatology. Incorporation of amyloid imaging in AD consensus diagnostic criteria highlight the importance of such findings [94]. Furthermore, it may be proven elusive in early detection of disease pathology, disease monitoring and as a biomarker in disease-modifying trials with treatment targeting amyloid deposition. In DLB apart from α-synuclein aggregation, in some cases, pathology is also characterized by amyloid-β and tau deposition [95, 96]. The concurrence of the above-mentioned events leads to greater cognitive impairment [97].
Subsequently, imaging amyloid-β and tau deposition could potentially elucidate the association between AD-related pathology and α-synuclein aggregation. [11C]-Pittsburgh compound B ([11C]PiB) has been the most used radioligand to assess amyloid-β deposition in patients with DLB. Patients with DLB have shown increased [11C]PiB retention when compared to patients with PD or PDD and reduced retention when compared to patients with AD [67]. However, although the load of amyloid-β deposition cannot distinguish DLB from AD, it can be associated with the pace of cognitive decline in DLB patients [98, 99]. Other studies have associated amyloid pathology to the time-onset of cognitive features when related to parkinsonism [62]. Meta-analyses highlighted that 68% of patients with a diagnosis of probable DLB harbor abnormal [11C]PiB retention [67, 100]. Regarding differences between DLB and PDD, it has been demonstrated that cortical amyloid-β burden is significantly high in DLB patients, which is comparable to amyloid-β retention in AD, but conversely to PDD patients where amyloid-β pathology is scarce [62]. Dementia severity has been to shown to be trivial in the differential load of amyloid-β between DLB and PDD, with amyloid deposition possessing the ability to differentiate DLB and PDD, despite their overwhelming overlap in clinical, neuropathologic and neuropsychologic features [94]. Gomperts et al., through measuring [11C]PiB retention in Lewy body diseases such as DLB, PDD, PD and PD-MCI, found that amyloid-β burden was higher in DLB subjects compared to the other groups, with amyloid deposits being associated to cognitive impairment exclusively in DLB [97]. The early amyloid burden in DLB, comparative to PDD, may account for the variability in onset of dementia and parkinsonism between the two conditions. However, it is important to note that [11C]PiB binds to amyloid fibrils, but not soluble amyloid oligomers, thus the possibility remains that both DLB and PDD have high levels of toxic amyloid oligomers, which could potentially underlie cognitive impairment in both conditions. Notably, [11C]PiB retention patients with probable DLB or PDD, tend to have a similar pattern of cortical atrophy in MRI to patients with AD [101]. A recent study comparing [11C]PiB binding to GM atrophy rates concluded that higher retention at baseline was correlated to increase loss of GM, greater ventricular expansion and cognitive impairment [101]. In concordance with novel therapeutic strategies in AD, where amyloid pathology is targeted, amyloid imaging will have an upgraded role when anti-amyloid treatments are available for DLB patients as well.
Tau imaging
The in vivo evaluation of tau pathology in DLB has been lacking until recently. The radioligand fluorine 18-labeled AV-1451 ([18F]AV-1451), also known as [18F]T807, has been proven suitable to assess tau deposition. Pathological studies have confirmed the predisposition of [18F]T807 for tau protein in neurofibrillary tangles instead of amyloid-β plaques or α-synuclein in Lewy bodies [102]. A recent study highlighted that cortical [18F]AV-1451 uptake was highly variable and greater than in the controls, especially in the inferior temporal gyrus and precuneus [102]. Furthermore, increased binding in these regions was found to be associated with cognitive impairment, as measured by the mini-mental state examination (MMSE) and the Clinical Dementia Rating scale [102]. These finding indicate a role for tau pathology in DLB pathogenesis. A subsequent larger [18F]AV-1451 PET imaging study reported that [18F]AV-1451 uptake was substantively more extensive and severe in AD compared to DLB patients [103]. [18F]AV-1451 uptake within the medial temporal lobe completely discriminated AD dementia from probable DLB, with AD exhibiting highest medial temporal uptake and DLB exhibiting the lowest. Probable DLB subjects had higher [18F]AV-1451 uptake in the posterior temporoparietal and occipital cortex compared to healthy controls, though no correlations were found between uptake in these regions and clinical measures such as motor parkinsonism, visual hallucinations, cognition or the presence of REM sleep behavior disorder (RBD). Global cortical [11C]PiB uptake, a marker of amyloid-β, was associated with elevated posterior temporoparietal and occipital [18F]AV-1451 uptake, indicating an atypical pattern of tau deposition in probable DLB [103]. Generally, there appears to be a gradient of increasing tau binding: from absent to minimal tau binding in cognitively normal PD, to low tau binding in PD patients with cognitive impairment, to intermediate tau binding in DLB and very high tau binding in AD [102, 104].
Alpha-synuclein imaging
Pathological SNCA is detected in various forms, such as fibrils, Lewy bodies and oligomers. Moreover, SNCA deposits are abundant in other misfolded proteins, including tau and amyloid [105]. Thus, a radiotracer with high selectivity for SNCA over tau and amyloid is required to provide adequate accuracy. Other key features of a potential radiotracer include high affinity for SNCA aggregates, high penetration in the brain and prompt clearance.
Several potential compounds with a desirable profile and acceptable characteristics have been identified and the production of an accurate radiotracer for SNCA remains the greatest challenge of the neuroimaging community in movement disorders [106]. One of the first compounds that was tested in vitro was the benzoxazole BF227 [107]. Although [18F]BF227 harbored high affinity for amyloid and low affinity for SNCA in brain tissues, it was also evaluated in vivo in a cohort of MSA patients without fully overcoming interpretation issues [108]. A group of phenothiazine derivatives has also been investigated in animal studies, as potential compounds, due to their moderate selectivity for SNCA in PD brains [109]. [18F]WC-58a harbored a promising selectivity and affinity for synthetic SNCA fibrils; however, it proved to be too lipophilic with a slow clearance [110].
The development of a reliable SNCA radioligand is an unmet need regarding in-depth cohort stratification, monitoring disease progression and designing experimental treatments for synucleinopathies. The presence of incidental Lewy body disease among elderly is a caveat regarding the diagnostic utility of SNCA-PET. However, the capability of in vivo quantification could provide a valuable tool, especially when combined with other modalities to understand the full spectrum and progression of overlapping proteinopathies.
Sensitivity and specificity of molecular imaging modalities in pathologically proven DLB cases
[123I]-FP-CIT-SPECT
The importance of [123I]FP-CIT-SPECT in the differential diagnosis of DLB and non-Lewy body dementias has been extensively elucidated and is appreciated in the clinical setting [111].
Class I evidence have been provided regarding the application of [123I]FP-CIT-SPECT in discriminating DLB patients [112]. However, results should be replicated with patients recruited from different clinical settings. Reduced uptake yields a respectable diagnostic accuracy in discriminating DLB from AD. Alas, regarding differential diagnosis with atypical parkinsonian syndromes and frontotemporal dementia, the utility of [123I]FP-CIT-SPECT is limited [86, 113, 114].
There are scarce studies evaluating [123I]FP-CIT-SPECT alongside post-mortem tissue in DLB (Table 4). Among all cohorts, [123I]FP-CIT-SPECT exhibits higher sensitivity and specificity when compared to clinical diagnosis. Vascular lesions in the substantia nigra have been reported as a cause of false positive results [115]. Although positive scans have been reported in PSP, FTLD and CBD, diagnosis can typically be made on distinct clinical characteristics. However, Thomas et al. have reported two false positive cases with features of parkinsonism and a clinical diagnosis of DLB; post-mortem diagnosis revealed either AD or FTLD features without evidence of SNCA pathology in the substantia nigra [112]. The authors also identified six cases of false negative scans; three of the cases had a clinical diagnosis of AD at baseline without any signs of parkinsonism. At post-mortem examination, they harbored a mixed picture of AD and DLB features. The other three cases were retrospectively reassessed and actually fulfilled criteria for probable DLB. Hence, although [123I]FP-CIT-SPECT harbors a suitable accuracy, absence of an abnormal scan cannot fully exclude the presence of DLB. This discrepancy could be explained either by the fact that [123I]FP-CIT-SPECT measures the effect of SNCA in neurons and not the deposition of SNCA per se; thus cortical and striatal pathology might be evident without substantial nigrostriatal neuronal degeneration.
Amyloid imaging
A study by Albin et al., combining amyloid and dopamine terminal PET imaging, revealed that imaging classifications were concordant with neuropathological diagnostic classifications in 33/36 cases (91.7%) [116]. Of three cases with discordant imaging-pathological classification, one had a clinical and imaging diagnosis of DLB, but a pathological diagnosis of AD. However, alpha-synuclein immunoreactive Lewy body inclusions were present in the midbrain, thus suggestive of mixed AD-DLB pathology. The other discordant subject was classified as DLB via imaging, but within the frontal cortex and hippocampus, had transactive response DNA binding protein 43 kDa (TDP-43)-immunoreactive neurites. This was particularly unusual given that this case exhibited unilateral striatal loss of [11C]DTBZ, a marker of striatal dopamine terminal integrity. Although 8.3% of cases differed in diagnostic classifications based on neuroimaging and histopathology, this was an improvement compared to their previous studies, which demonstrated that ~ 35% of cases had discordant expert clinical consensus and imaging classifications [117, 118]. Therefore, this combined imaging approach may be useful in establishing more accurate markers for differentiating dementias.
[18F]FDG PET
Patterns of cerebral glucose metabolism in DLB have been reported to encompass the ability to differentiate DLB from other forms of dementia. Although DLB has shown to exhibit widespread glucose hypometabolism across cortical regions, metabolic reduction has been shown to be most prominent within the visual association cortex. Through looking at metabolism within this region, DLB can be distinguished from AD with a sensitivity of 86% and specificity of 91% [119]. Although these authors only scanned one patient with autopsy-confirmed DLB diagnosis, postmortem results from 17 DLB brains revealed a distinct and extensive white matter spongiform change with coexisting gliosis throughout cerebral white matter. These changes were consistently and pronouncedly observed within the occipital lobe, with the severity of the regional spongiform change mainly corresponding to the regional differences in patterns of reduced glucose metabolism illustrated by living AD and DLB patients [119]. This study is in corroboration with findings reported by Minoshima et al., who revealed that autopsy-confirmed AD and DLB patients exhibited regional metabolic reductions, specifically within posterior cingulate, parietotemporal association, and frontal association cortex. DLB cases, in particular, demonstrated significant metabolic reductions within the occipital cortex, specifically within the primary visual cortex, which had the ability to distinguish DLB from AD with a specificity of 80% and sensitivity of 90% [120]. Furthermore, patients who were initially clinically diagnosed with probable AD, but later fulfilled the clinical criteria for DLB, demonstrated hypometabolism within the primary visual cortex at higher frequencies, which often preceded the manifestation of several DLB symptoms [120]. Although the authors of these studies argue that [18F]FDG PET may be a useful tool to distinguish DLB from other dementias, Albin et al. demonstrated that ~ 30% of classifications, based on glucose metabolism, differed from final neuropathological diagnoses in a cohort of DLB, AD and FTD who underwent PET imaging and subsequent autopsy [116]. They reported 2 cases where [18F]FDG PET classification was AD but pathological verification was DLB, though combined amyloid and dopaminergic terminal PET imaging correctly identified the pathological diagnosis [116]. Therefore, the authors argued that classifications based on [18F]FDG PET are less precise, with misclassifications ascribed to [18F]FDG PET being due to the absence of occipital metabolic deficits in a substantial proportion of DLB patients [121].
A proposed [18F]FDG PET imaging feature of DLB is the cingulate island sign, which refers to the sparing of the posterior cingulate relative to the precuneus and cuneus. This sign is said to be useful for an accurate diagnosis of DLB, given that it is specific, with a reasonable sensitivity [66]. Studies assessing this sign in clinically diagnosed DLB have revealed that the cingulate island sign metabolism, as measured by [18F]FDG PET, is highly specific for detecting DLB, with a specificity of 100% and sensitivity ranging from 62 to 86% [66]. In this study, 4/14 subjects had autopsy-confirmed DLB, with the others being followed clinically for several years and their diagnosis remaining unchanged. Similarly, the cingulate island sign metabolism is higher in DLB patients compared to AD, independent of amyloid load [72]. Patients who exhibited the cingulate island sign were more likely to be classified as having high or intermediate probability of DLB pathology, receiving a clinical diagnosis of DLB. Furthermore, a higher cingulate island sign ratio was associated with a lower burden of neurofibrillary tangles. 2 subjects who had the lowest cingulate island sign ratio where clinically diagnosed with DLB, but at autopsy, exhibited high likelihood of AD pathology without Lewy body pathology. Taken together, these results indicate that a reduction in cingulate island sign ratio is associated with high burden of AD-type neurofibrillary tangles, therefore ‘pure’ DLB would present with the typical cingulate island sign. This is incredibly important, as the convergence and co-occurrence of AD and DLB pathology is common, with ‘pure’ DLB accounting for no more than a third of all DLB cases and possibly 10% of all clinical dementia cases. This was demonstrated by Barker et al., who identified DLB in 14–26% of dementia cases, with the ‘pure’ form of DLB accounting for 0–19% of dementia patients [122]. Therefore, whilst helpful, the cingulate island sign will not very sensitive or specific for DLB in other pathological series.
Conclusions
DLB is a common dementia in older patients and differential diagnosis with AD and especially PDD can be challenging. Well-established neuroimaging modalities such as [123I]FP-CIT-SPECT and [123I]MIBG can be extremely useful in adding diagnostic accuracy between DLB and AD but not with PD-MCI and PDD or atypical parkinsonian syndromes. The application of novel radioligands targeting pathways relevant to underlying pathophysiology, can provide valuable tools in exploring molecular pathology. Furthermore, precise quantification of tau pathology and the possibility of a tracer targeting α-synuclein will further expand insights and potentially harbor innovative therapeutic opportunities.
References
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22(12):1689–1707. https://doi.org/10.1002/mds.21507 quiz 1837.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Seidel K, Mahlke J, Siswanto S, Kruger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf HW, den Dunnen W, Rub U (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25(2):121–135. https://doi.org/10.1111/bpa.12168
Elahi FM, Miller BL (2017) A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol 13(8):457–476. https://doi.org/10.1038/nrneurol.2017.96
Zahirovic I, Wattmo C, Torisson G, Minthon L, Londos E (2016) Prevalence of dementia with Lewy body symptoms: a cross-sectional study in 40 Swedish nursing homes. J Am Med Dir Assoc 17(8):706–711. https://doi.org/10.1016/j.jamda.2016.03.017
Aarsland D, Perry R, Larsen JP, McKeith IG, O’Brien JT, Perry EK, Burn D, Ballard CG (2005) Neuroleptic sensitivity in Parkinson’s disease and parkinsonian dementias. J Clin Psychiatry 66(5):633–637
Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M (2008) EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 131(Pt 3):690–705. https://doi.org/10.1093/brain/awm322
Lim X, Yeo JM, Green A, Pal S (2013) The diagnostic utility of cerebrospinal fluid alpha-synuclein analysis in dementia with Lewy bodies—a systematic review and meta-analysis. Parkinsonism Relat Disord 19(10):851–858. https://doi.org/10.1016/j.parkreldis.2013.06.008
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89(1):88–100. https://doi.org/10.1212/WNL.0000000000004058
Filippi M, Agosta F, Barkhof F, Dubois B, Fox NC, Frisoni GB, Jack CR, Johannsen P, Miller BL, Nestor PJ, Scheltens P, Sorbi S, Teipel S, Thompson PM, Wahlund LO, European Federation of the Neurologic S (2012) EFNS task force: the use of neuroimaging in the diagnosis of dementia. Eur J Neurol 19(12):e131–e140. https://doi.org/10.1111/j.1468-1331.2012.03859.x 1487–1501.
Kemp PM, Holmes C (2007) Imaging in dementia with Lewy bodies: a review. Nucl Med Commun 28(7):511–519. https://doi.org/10.1097/MNM.0b013e3281e20a12
Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O’Brien J (1999) White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry 67(1):66–72
O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST (2003) Vascular cognitive impairment. Lancet Neurol 2(2):89–98
Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, McKeith IG, Scheltens P, Barkhof F, O’Brien JT (2002) Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage 17(2):618–630
Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O’Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C, Barkhof F (2003) A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. Neuroimage 18(4):895–907
Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, Kalaria RN, O’Brien JT (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132(Pt 1):195–203. https://doi.org/10.1093/brain/awn298
Watson R, Colloby SJ, Blamire AM, O’Brien JT (2015) Assessment of regional gray matter loss in dementia with Lewy bodies: a surface-based MRI analysis. Am J Geriatr Psychiatry 23(1):38–46. https://doi.org/10.1016/j.jagp.2014.07.005
Lebedev AV, Westman E, Beyer MK, Kramberger MG, Aguilar C, Pirtosek Z, Aarsland D (2013) Multivariate classification of patients with Alzheimer’s and dementia with Lewy bodies using high-dimensional cortical thickness measurements: an MRI surface-based morphometric study. J Neurol 260(4):1104–1115. https://doi.org/10.1007/s00415-012-6768-z
Ballmaier M, O’Brien JT, Burton EJ, Thompson PM, Rex DE, Narr KL, McKeith IG, DeLuca H, Toga AW (2004) Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer’s disease using cortical pattern matching: diagnosis and gender effects. Neuroimage 23(1):325–335. https://doi.org/10.1016/j.neuroimage.2004.04.026
Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT (2000) MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 54(6):1304–1309
Chow N, Aarsland D, Honarpisheh H, Beyer MK, Somme JH, Elashoff D, Rongve A, Tysnes OB, Thompson PM, Apostolova LG (2012) Comparing hippocampal atrophy in Alzheimer’s dementia and dementia with lewy bodies. Dement Geriatr Cogn Disord 34(1):44–50. https://doi.org/10.1159/000339727
Watson R, O’Brien JT, Barber R, Blamire AM (2012) Patterns of gray matter atrophy in dementia with Lewy bodies: a voxel-based morphometry study. Int Psychogeriatr 24(4):532–540. https://doi.org/10.1017/S1041610211002171
Hayashi H, Kawakatsu S, Suzuki A, Shibuya Y, Kobayashi R, Sato C, Otani K (2012) Application of the VSRAD, a specific and sensitive voxel-based morphometry, to comparison of entorhinal cortex atrophy between dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord 34(5–6):328–331. https://doi.org/10.1159/000345792
Delli Pizzi S, Franciotti R, Bubbico G, Thomas A, Onofrj M, Bonanni L (2016) Atrophy of hippocampal subfields and adjacent extrahippocampal structures in dementia with Lewy bodies and Alzheimer’s disease. Neurobiol Aging 40:103–109. https://doi.org/10.1016/j.neurobiolaging.2016.01.010
Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, Knopman DS, Petersen RC, Benarroch EE, Josephs KA, Jack CR Jr (2007) Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain 130(Pt 3):708–719. https://doi.org/10.1093/brain/awl388
Watson R, Blamire AM, Colloby SJ, Wood JS, Barber R, He J, O’Brien JT (2012) Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 79(9):906–914. https://doi.org/10.1212/WNL.0b013e318266fc51
Shams S, Fallmar D, Schwarz S, Wahlund LO, van Westen D, Hansson O, Larsson EM, Haller S (2017) MRI of the swallow tail sign: a useful marker in the diagnosis of Lewy body dementia? AJNR Am J Neuroradiol 38(9):1737–1741. https://doi.org/10.3174/ajnr.A5274
Kamagata K, Nakatsuka T, Sakakibara R, Tsuyusaki Y, Takamura T, Sato K, Suzuki M, Hori M, Kumamaru KK, Inaoka T, Aoki S, Terada H (2017) Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology 59(1):89–98. https://doi.org/10.1007/s00234-016-1773-z
Mak E, Su L, Williams GB, O’Brien JT (2014) Neuroimaging characteristics of dementia with Lewy bodies. Alzheimers Res Ther 6(2):18. https://doi.org/10.1186/alzrt248
Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127(Pt 4):791–800. https://doi.org/10.1093/brain/awh088
Beyer MK, Larsen JP, Aarsland D (2007) Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology 69(8):747–754. https://doi.org/10.1212/01.wnl.0000269666.62598.1c
Lee JE, Park B, Song SK, Sohn YH, Park HJ, Lee PH (2010) A comparison of gray and white matter density in patients with Parkinson’s disease dementia and dementia with Lewy bodies using voxel-based morphometry. Mov Disord 25(1):28–34. https://doi.org/10.1002/mds.22858
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, Calopa M, Jauma S, Juncadella M, Junque C (2009) Correlations between gray matter reductions and cognitive deficits in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord 24(12):1740–1746. https://doi.org/10.1002/mds.22488
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, Calopa M, Jauma S, Juncadella M, Junque C (2010) Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord 25(5):615–622. https://doi.org/10.1002/mds.22873
Galvin JE, Price JL, Yan Z, Morris JC, Sheline YI (2011) Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology 76(21):1797–1803. https://doi.org/10.1212/WNL.0b013e31821ccc83
Franciotti R, Falasca NW, Bonanni L, Anzellotti F, Maruotti V, Comani S, Thomas A, Tartaro A, Taylor JP, Onofrj M (2013) Default network is not hypoactive in dementia with fluctuating cognition: an Alzheimer disease/dementia with Lewy bodies comparison. Neurobiol Aging 34(4):1148–1158. https://doi.org/10.1016/j.neurobiolaging.2012.09.015
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101(13):4637–4642. https://doi.org/10.1073/pnas.0308627101
Kenny ER, O’Brien JT, Firbank MJ, Blamire AM (2013) Subcortical connectivity in dementia with Lewy bodies and Alzheimer’s disease. Br J Psychiatry 203(3):209–214. https://doi.org/10.1192/bjp.bp.112.108464
Schumacher J, Peraza LR, Firbank M, Thomas AJ, Kaiser M, Gallagher P, O’Brien JT, Blamire AM, Taylor JP (2018) Functional connectivity in dementia with Lewy bodies: A within- and between-network analysis. Hum Brain Mapp 39(3):1118–1129. https://doi.org/10.1002/hbm.23901
Sauer J, ffytche DH, Ballard C, Brown RG, Howard R (2006) Differences between Alzheimer’s disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain 129(Pt 7):1780–1788. https://doi.org/10.1093/brain/awl102
Taylor JP, Firbank MJ, He J, Barnett N, Pearce S, Livingstone A, Vuong Q, McKeith IG, O’Brien JT (2012) Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Br J Psychiatry 200(6):491–498. https://doi.org/10.1192/bjp.bp.111.099432
Rektorova I, Krajcovicova L, Marecek R, Mikl M (2012) Default mode network and extrastriate visual resting state network in patients with Parkinson’s disease dementia. Neurodegener Dis 10(1–4):232–237. https://doi.org/10.1159/000334765
Seibert TM, Murphy EA, Kaestner EJ, Brewer JB (2012) Interregional correlations in Parkinson disease and Parkinson-related dementia with resting functional MR imaging. Radiology 263(1):226–234. https://doi.org/10.1148/radiol.12111280
Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, Corbo D, Cirillo G, Barone P, Tedeschi G (2012) Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79(23):2226–2232. https://doi.org/10.1212/WNL.0b013e31827689d6
Baggio HC, Segura B, Sala-Llonch R, Marti MJ, Valldeoriola F, Compta Y, Tolosa E, Junque C (2015) Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum Brain Mapp 36(1):199–212. https://doi.org/10.1002/hbm.22622
Kenny ER, Blamire AM, Firbank MJ, O’Brien JT (2012) Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer’s disease. Brain 135(Pt 2):569–581. https://doi.org/10.1093/brain/awr327
Lowther ER, O’Brien JT, Firbank MJ, Blamire AM (2014) Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res 223(3):192–201. https://doi.org/10.1016/j.pscychresns.2014.06.004
Peraza LR, Kaiser M, Firbank M, Graziadio S, Bonanni L, Onofrj M, Colloby SJ, Blamire A, O’Brien J, Taylor JP (2014) fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. Neuroimage Clin 4:558–565. https://doi.org/10.1016/j.nicl.2014.03.013
Peraza LR, Colloby SJ, Firbank MJ, Greasy GS, McKeith IG, Kaiser M, O’Brien J, Taylor JP (2015) Resting state in Parkinson’s disease dementia and dementia with Lewy bodies: commonalities and differences. Int J Geriatr Psychiatry 30(11):1135–1146. https://doi.org/10.1002/gps.4342
Mak E, Su L, Williams GB, Watson R, Firbank MJ, Blamire AM, O’Brien JT (2015) Progressive cortical thinning and subcortical atrophy in dementia with Lewy bodies and Alzheimer’s disease. Neurobiol Aging 36(4):1743–1750. https://doi.org/10.1016/j.neurobiolaging.2014.12.038
Williams MM, Xiong C, Morris JC, Galvin JE (2006) Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology 67(11):1935–1941. https://doi.org/10.1212/01.wnl.0000247041.63081.98
Shimizu S, Hanyu H, Hirao K, Sato T, Iwamoto T, Koizumi K (2008) Value of analyzing deep gray matter and occipital lobe perfusion to differentiate dementia with Lewy bodies from Alzheimer’s disease. Ann Nucl Med 22(10):911–916. https://doi.org/10.1007/s12149-008-0193-5
Watson R, Colloby SJ, Blamire AM, O’Brien JT (2016) Subcortical volume changes in dementia with Lewy bodies and Alzheimer’s disease. A comparison with healthy aging. Int Psychogeriatr 28(4):529–536. https://doi.org/10.1017/S1041610215001805
Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, Burn D, Johnson M, Perry RH, McKeith IG, Ballard C, Perry EK (1999) Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain 122(Pt 8):1449–1468
Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O’Brien JT (2000) MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry 15(10):911–916
Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT (2006) Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry 14(10):842–849. https://doi.org/10.1097/01.JGP.0000236596.56982.1c
De Reuck J, Deramecourt V, Auger F, Durieux N, Cordonnier C, Devos D, Defebvre L, Moreau C, Caparros-Lefebvre D, Bordet R, Maurage CA, Pasquier F, Leys D (2014) Post-mortem 7.0-tesla magnetic resonance study of cortical microinfarcts in neurodegenerative diseases and vascular dementia with neuropathological correlates. J Neurol Sci 346(1–2):85–89. https://doi.org/10.1016/j.jns.2014.07.061
Fukui T, Oowan Y, Yamazaki T, Kinno R (2013) Prevalence and clinical implication of microbleeds in dementia with lewy bodies in comparison with microbleeds in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra 3(1):148–160. https://doi.org/10.1159/000351423
Ballard C, O’Brien J, Barber B, Scheltens P, Shaw F, McKeith I, Kenny RA (2000) Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann N Y Acad Sci 903:442–445
Barkhof F, Polvikoski TM, van Straaten EC, Kalaria RN, Sulkava R, Aronen HJ, Niinisto L, Rastas S, Oinas M, Scheltens P, Erkinjuntti T (2007) The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology 69(15):1521–1527. https://doi.org/10.1212/01.wnl.0000277459.83543.99
Harper L, Fumagalli GG, Barkhof F, Scheltens P, O’Brien JT, Bouwman F, Burton EJ, Rohrer JD, Fox NC, Ridgway GR, Schott JM (2016) MRI visual rating scales in the diagnosis of dementia: evaluation in 184 post-mortem confirmed cases. Brain 139(Pt 4):1211–1225. https://doi.org/10.1093/brain/aww005
Petrou M, Dwamena BA, Foerster BR, MacEachern MP, Bohnen NI, Muller ML, Albin RL, Frey KA (2015) Amyloid deposition in Parkinson’s disease and cognitive impairment: a systematic review. Mov Disord 30(7):928–935. https://doi.org/10.1002/mds.26191
Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, Gunter JL, Senjem ML, Vemuri P, Smith GE, Geda YE, Graff-Radford J, Knopman DS, Petersen RC, Parisi JE, Dickson DW, Jack CR Jr, Kantarci K (2015) Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 36(1):452–461. https://doi.org/10.1016/j.neurobiolaging.2014.07.005
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Hanyu H, Shimizu S, Hirao K, Kanetaka H, Sakurai H, Iwamoto T, Koizumi K, Abe K (2006) Differentiation of dementia with Lewy bodies from Alzheimer’s disease using Mini-Mental State Examination and brain perfusion SPECT. J Neurol Sci 250(1–2):97–102. https://doi.org/10.1016/j.jns.2006.07.007
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, Bradshaw J, Merory J, Woodward M, Hopwood M, Rowe CC (2009) The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med 50(10):1638–1645. https://doi.org/10.2967/jnumed.109.065870
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhury KR, Masters CL, Brooks DJ (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79(12):1331–1338. https://doi.org/10.1136/jnnp.2007.127878
Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R (2010) Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74(11):885–892. https://doi.org/10.1212/WNL.0b013e3181d55f61
Perneczky R, Drzezga A, Boecker H, Forstl H, Kurz A, Haussermann P (2008) Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord 25(6):531–538. https://doi.org/10.1159/000132084
Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, McKeith IG, O’Brien JT (2001) Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 56(5):643–649
Liu S, Wang XD, Wang Y, Shi Z, Cai L, Liu S, Han T, Zhou Y, Wang X, Gao S, Ji Y (2017) Clinical and neuroimaging characteristics of Chinese dementia with Lewy bodies. PLoS One 12(3):e0171802. https://doi.org/10.1371/journal.pone.0171802
Graff-Radford J, Murray ME, Lowe VJ, Boeve BF, Ferman TJ, Przybelski SA, Lesnick TG, Senjem ML, Gunter JL, Smith GE, Knopman DS, Jack CR Jr, Dickson DW, Petersen RC, Kantarci K (2014) Dementia with Lewy bodies: basis of cingulate island sign. Neurology 83(9):801–809. https://doi.org/10.1212/WNL.0000000000000734
Iizuka T, Kameyama M (2016) Cingulate island sign on FDG-PET is associated with medial temporal lobe atrophy in dementia with Lewy bodies. Ann Nucl Med 30(6):421–429. https://doi.org/10.1007/s12149-016-1076-9
Brigo F, Turri G, Tinazzi M (2015) 123I-FP-CIT SPECT in the differential diagnosis between dementia with Lewy bodies and other dementias. J Neurol Sci 359(1–2):161–171. https://doi.org/10.1016/j.jns.2015.11.004
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, Aarsland D, McKeith IG (2004) Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol 61(6):919–925. https://doi.org/10.1001/archneur.61.6.919
Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G, Ince PG, Perry R, McKeith I, Katona CL (2007) Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 78(11):1176–1181. https://doi.org/10.1136/jnnp.2006.110122
O’Brien JT, McKeith IG, Walker Z, Tatsch K, Booij J, Darcourt J, Marquardt M, Reininger C, Group DLBS. (2009) Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry 194(1):34–39. https://doi.org/10.1192/bjp.bp.108.052050
Papathanasiou ND, Boutsiadis A, Dickson J, Bomanji JB (2012) Diagnostic accuracy of (1)(2)(3)I-FP-CIT (DaTSCAN) in dementia with Lewy bodies: a meta-analysis of published studies. Parkinsonism Relat Disord 18(3):225–229. https://doi.org/10.1016/j.parkreldis.2011.09.015
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, Padovani A, Giubbini R, Bonuccelli U, Volterrani D, Holmes C, Kemp P, Tabet N, Meyer I, Reininger C, Group DLBS. (2007) Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 6(4):305–313. https://doi.org/10.1016/S1474-4422(07)70057-1
McCleery J, Morgan S, Bradley KM, Noel-Storr AH, Ansorge O, Hyde C (2015) Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev 1:CD010633. https://doi.org/10.1002/14651858.CD010633.pub2
Shimizu S, Hanyu H, Kanetaka H, Iwamoto T, Koizumi K, Abe K (2005) Differentiation of dementia with Lewy bodies from Alzheimer’s disease using brain SPECT. Dement Geriatr Cogn Disord 20(1):25–30. https://doi.org/10.1159/000085070
Colloby SJ, Firbank MJ, Pakrasi S, Lloyd JJ, Driver I, McKeith IG, Williams ED, O’Brien JT (2008) A comparison of 99mTc-exametazime and 123I-FP-CIT SPECT imaging in the differential diagnosis of Alzheimer’s disease and dementia with Lewy bodies. Int Psychogeriatr 20(6):1124–1140. https://doi.org/10.1017/S1041610208007709
Colloby SJ, Fenwick JD, Williams ED, Paling SM, Lobotesis K, Ballard C, McKeith I, O’Brien JT (2002) A comparison of (99 m)Tc-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. Eur J Nucl Med Mol Imaging 29(5):615–622. https://doi.org/10.1007/s00259-002-0778-5
Chang CC, Liu JS, Chang YY, Chang WN, Chen SS, Lee CH (2008) (99 m)Tc-ethyl cysteinate dimer brain SPECT findings in early stage of dementia with Lewy bodies and Parkinson’s disease patients: a correlation with neuropsychological tests. Eur J Neurol 15(1):61–65. https://doi.org/10.1111/j.1468-1331.2007.02001.x
Takaya S, Sawamoto N, Okada T, Okubo G, Nishida S, Togashi K, Fukuyama H, Takahashi R (2017) Differential diagnosis of parkinsonian syndromes using dopamine transporter and perfusion SPECT. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2017.11.333
Morgan S, Kemp P, Booij J, Costa DC, Padayachee S, Lee L, Barber C, Carter J, Walker Z (2012) Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J Neurol Neurosurg Psychiatry 83(11):1063–1070. https://doi.org/10.1136/jnnp-2012-302577
Inui Y, Toyama H, Manabe Y, Sarai M, Iwata N (2014) Comparison of (123)I-MIBG myocardial scintigraphy, brain perfusion SPECT, and voxel-based MRI morphometry for distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Ann Nucl Med 28(8):796–804. https://doi.org/10.1007/s12149-014-0873-2
Hanyu H, Shimizu S, Hirao K, Kanetaka H, Iwamoto T, Chikamori T, Usui Y, Yamashina A, Koizumi K, Abe K (2006) Comparative value of brain perfusion SPECT and [(123)I]MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 33(3):248–253. https://doi.org/10.1007/s00259-005-1921-x
Oda H, Ishii K, Terashima A, Shimada K, Yamane Y, Kawasaki R, Ohkawa S (2013) Myocardial scintigraphy may predict the conversion to probable dementia with Lewy bodies. Neurology 81(20):1741–1745. https://doi.org/10.1212/01.wnl.0000435553.67953.81
King AE, Mintz J, Royall DR (2011) Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord 26(7):1218–1224. https://doi.org/10.1002/mds.23659
Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, Fukasawa R, Umahara T, Sakurai H, Hanyu H (2016) Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43(1):184–192. https://doi.org/10.1007/s00259-015-3146-y
Yoshita M, Arai H, Arai H, Arai T, Asada T, Fujishiro H, Hanyu H, Iizuka O, Iseki E, Kashihara K, Kosaka K, Maruno H, Mizukami K, Mizuno Y, Mori E, Nakajima K, Nakamura H, Nakano S, Nakashima K, Nishio Y, Orimo S, Samuraki M, Takahashi A, Taki J, Tokuda T, Urakami K, Utsumi K, Wada K, Washimi Y, Yamasaki J, Yamashina S, Yamada M (2015) Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One 10(3):e0120540. https://doi.org/10.1371/journal.pone.0120540
Treglia G, Cason E (2012) Diagnostic performance of myocardial innervation imaging using MIBG scintigraphy in differential diagnosis between dementia with lewy bodies and other dementias: a systematic review and a meta-analysis. J Neuroimaging 22(2):111–117. https://doi.org/10.1111/j.1552-6569.2010.00532.x
Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71(12):903–910. https://doi.org/10.1212/01.wnl.0000326146.60732.d6
Sarro L, Senjem ML, Lundt ES, Przybelski SA, Lesnick TG, Graff-Radford J, Boeve BF, Lowe VJ, Ferman TJ, Knopman DS, Comi G, Filippi M, Petersen RC, Jack CR Jr, Kantarci K (2016) Amyloid-beta deposition and regional grey matter atrophy rates in dementia with Lewy bodies. Brain 139(Pt 10):2740–2750. https://doi.org/10.1093/brain/aww193
Donaghy P, Thomas AJ, O’Brien JT (2015) Amyloid PET Imaging in Lewy body disorders. Am J Geriatr Psychiatry 23(1):23–37. https://doi.org/10.1016/j.jagp.2013.03.001
Gomperts SN, Locascio JJ, Marquie M, Santarlasci AL, Rentz DM, Maye J, Johnson KA, Growdon JH (2012) Brain amyloid and cognition in Lewy body diseases. Mov Disord 27(8):965–973. https://doi.org/10.1002/mds.25048
Maetzler W, Liepelt I, Reimold M, Reischl G, Solbach C, Becker C, Schulte C, Leyhe T, Keller S, Melms A, Gasser T, Berg D (2009) Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis 34(1):107–112. https://doi.org/10.1016/j.nbd.2008.12.008
Gilman S, Koeppe RA, Little R, An H, Junck L, Giordani B, Persad C, Heumann M, Wernette K (2005) Differentiation of Alzheimer’s disease from dementia with Lewy bodies utilizing positron emission tomography with [18F]fluorodeoxyglucose and neuropsychological testing. Exp Neurol 191(Suppl 1):S95–S103. https://doi.org/10.1016/j.expneurol.2004.06.017
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL (2007) Imaging beta-amyloid burden in aging and dementia. Neurology 68(20):1718–1725. https://doi.org/10.1212/01.wnl.0000261919.22630.ea
Jokinen P, Scheinin N, Aalto S, Nagren K, Savisto N, Parkkola R, Rokka J, Haaparanta M, Roytta M, Rinne JO (2010) [(11)C]PIB-, [(18)F]FDG-PET and MRI imaging in patients with Parkinson’s disease with and without dementia. Parkinsonism Relat Disord 16(10):666–670. https://doi.org/10.1016/j.parkreldis.2010.08.021
Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, Vasdev N, Sperling R, Growdon JH, Dickerson BC, Johnson K (2016) Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases. JAMA Neurol 73(11):1334–1341. https://doi.org/10.1001/jamaneurol.2016.3338
Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, Lesnick TG, Spychalla AJ, Gunter JL, Fields JA, Graff-Radford J, Ferman TJ, Jones DT, Murray ME, Knopman DS, Jack CR Jr, Petersen RC (2017) AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 81(1):58–67. https://doi.org/10.1002/ana.24825
Hansen AK, Damholdt MF, Fedorova TD, Knudsen K, Parbo P, Ismail R, Ostergaard K, Brooks DJ, Borghammer P (2017) In Vivo cortical tau in Parkinson’s disease using 18F-AV-1451 positron emission tomography. Mov Disord 32(6):922–927. https://doi.org/10.1002/mds.26961
Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS (2012) Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol 69(10):1326–1331. https://doi.org/10.1001/archneurol.2012.1608
Yu L, Cui J, Padakanti PK, Engel L, Bagchi DP, Kotzbauer PT, Tu Z (2012) Synthesis and in vitro evaluation of alpha-synuclein ligands. Bioorg Med Chem 20(15):4625–4634. https://doi.org/10.1016/j.bmc.2012.06.023
Fodero-Tavoletti MT, Mulligan RS, Okamura N, Furumoto S, Rowe CC, Kudo Y, Masters CL, Cappai R, Yanai K, Villemagne VL (2009) In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol 617(1–3):54–58. https://doi.org/10.1016/j.ejphar.2009.06.042
Kikuchi A, Takeda A, Okamura N, Tashiro M, Hasegawa T, Furumoto S, Kobayashi M, Sugeno N, Baba T, Miki Y, Mori F, Wakabayashi K, Funaki Y, Iwata R, Takahashi S, Fukuda H, Arai H, Kudo Y, Yanai K, Itoyama Y (2010) In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain 133(Pt 6):1772–1778. https://doi.org/10.1093/brain/awq091
Zhang X, Jin H, Padakanti PK, Li J, Yang H, Fan J, Mach RH, Kotzbauer P, Tu Z (2014) Radiosynthesis and in vivo evaluation of two PET radioligands for imaging alpha-synuclein. Appl Sci (Basel) 4(1):66–78. https://doi.org/10.3390/app4010066
Chu W, Zhou D, Gaba V, Liu J, Li S, Peng X, Xu J, Dhavale D, Bagchi DP, d’Avignon A, Shakerdge NB, Bacskai BJ, Tu Z, Kotzbauer PT, Mach RH (2015) Design, synthesis, and characterization of 3-(Benzylidene)indolin-2-one derivatives as ligands for alpha-synuclein fibrils. J Med Chem 58(15):6002–6017. https://doi.org/10.1021/acs.jmedchem.5b00571
Grosset DG, Tatsch K, Oertel WH, Tolosa E, Bajaj N, Kupsch A, O’Brien JT, Seibyl J, Walker Z, Sherwin P, Chen C, Grachev ID (2014) Safety analysis of 10 clinical trials and for 13 years after first approval of ioflupane 123I injection (DaTscan). J Nucl Med 55(8):1281–1287. https://doi.org/10.2967/jnumed.114.138032
Thomas AJ, Attems J, Colloby SJ, O’Brien JT, McKeith I, Walker R, Lee L, Burn D, Lett DJ, Walker Z (2017) Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88(3):276–283. https://doi.org/10.1212/WNL.0000000000003512
Ba F, Martin WR (2015) Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord 21(2):87–94. https://doi.org/10.1016/j.parkreldis.2014.11.007
Seppi K, Scherfler C, Donnemiller E, Virgolini I, Schocke MF, Goebel G, Mair KJ, Boesch S, Brenneis C, Wenning GK, Poewe W (2006) Topography of dopamine transporter availability in progressive supranuclear palsy: a voxelwise [123I]beta-CIT SPECT analysis. Arch Neurol 63(8):1154–1160. https://doi.org/10.1001/archneur.63.8.1154
Walker RW, Walker Z (2009) Dopamine transporter single photon emission computerized tomography in the diagnosis of dementia with Lewy bodies. Mov Disord 24 Suppl 2:S754–S759. https://doi.org/10.1002/mds.22591
Albin RL, Fisher-Hubbard A, Shanmugasundaram K, Koeppe RA, Burke JF, Camelo-Piragua S, Lieberman AP, Giordani B, Frey KA (2015) Post-mortem evaluation of amyloid-dopamine terminal positron emission tomography dementia classifications. Ann Neurol 78(5):824–830. https://doi.org/10.1002/ana.24481
Burke JF, Albin RL, Koeppe RA, Giordani B, Kilbourn MR, Gilman S, Frey KA (2011) Assessment of mild dementia with amyloid and dopamine terminal positron emission tomography. Brain 134(Pt 6):1647–1657. https://doi.org/10.1093/brain/awr089
Albin RL, Burke JF, Koeppe RA, Giordani B, Gilman S, Frey KA (2013) Assessing mild cognitive impairment with amyloid and dopamine terminal molecular imaging. J Nucl Med 54(6):887–893. https://doi.org/10.2967/jnumed.112.112599
Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, Itoh M, Shin RW, Trojanowski JQ, Sasaki H (2000) Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol 162(2):247–256. https://doi.org/10.1006/exnr.2000.7342
Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE (2001) Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol 50(3):358–365
Kantarci K, Lowe VJ, Boeve BF, Weigand SD, Senjem ML, Przybelski SA, Dickson DW, Parisi JE, Knopman DS, Smith GE, Ferman TJ, Petersen RC, Jack CR Jr (2012) Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging 33(9):2091–2105. https://doi.org/10.1016/j.neurobiolaging.2011.09.024
Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R (2002) Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 16(4):203–212
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure statement
Ms Yousaf, Dr. Dervenoulas, Dr. Valkimadi and Prof. Politis report no disclosures.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yousaf, T., Dervenoulas, G., Valkimadi, PE. et al. Neuroimaging in Lewy body dementia. J Neurol 266, 1–26 (2019). https://doi.org/10.1007/s00415-018-8892-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8892-x