Abstract
Background
Treatment choice in multiple sclerosis (MS) is crucial for optimizing risk–benefit profile.
Objective
To assess fingolimod (FTY) effectiveness and identify baseline features associated to disease activity in a large Italian cohort of Relapsing–Remitting (RR) MS patients.
Methods
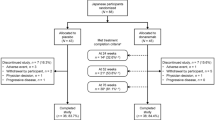
Three-hundred sixty-seven RRMS patients starting FTY treatment at San Raffaele Hospital (Milan-Italy) underwent clinical and MRI evaluations for 2 years. Treatment response was assessed considering the proportion of patients with no evidence of disease activity (NEDA) and recording the time to first relapse. Primary analyses were performed stratifying for Natalizumab (NTZ) treatment in the year before (NO_NTZ vs NTZ group), to account for post-NTZ reactivation.
Results
Almost half of patients were NEDA after 2 years, 53.4% in the NO_NTZ group and 36.2% in the NTZ group. Despite an opposite trend during the first 6–12 months, at 2-year follow-up the two groups were comparable for relapses and number of new/enlarging T2 and Gd-enhancing lesions. Baseline parameters of higher disease activity (ARR, Gd enhancing lesions and age at onset) were associated with increased likelihood of failing NEDA criteria or with shorter time to relapse (p < 0.05).
Conclusions
Our data strengthen FTY effectiveness in everyday clinical practice, even in patients switching from NTZ treatment. Baseline parameters of inflammatory activity are the most important prognostic factors for mid-term disease reactivation also during second-line treatment with FTY, providing hints on how to select therapies towards a more personalized management.


Similar content being viewed by others
References
Ransohoff RM, Hafler DA, Lucchinetti CF (2015) Multiple sclerosis-a quiet revolution. Nat Rev Neurol 11(3):134–142
Gafson A, Craner MJ, Matthews PM (2016) Personalised medicine for multiple sclerosis care. Mult Scler 23(3):362–369
Calabresi PA, Radue EW, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13(6):545–556
Kappos L, Radue EW, O’Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362(5):387–401
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362(5):402–415
Alping P, Frisell T, Novakova L et al (2016) Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 79(6):950–958
Braune S, Lang M, Bergmann A, Neuro Trans Data Study G (2016) Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 263(2):327–333
Frisell T, Forsberg L, Nordin N et al (2016) Comparative analysis of first-year fingolimod and natalizumab drug discontinuation among Swedish patients with multiple sclerosis. Mult Scler 22(1):85–93
Iaffaldano P, Lucisano G, Pozzilli C et al (2015) Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 138(Pt 11):3275–3286
Jokubaitis VG, Li V, Kalincik T et al (2014) Fingolimod after natalizumab and the risk of short-term relapse. Neurology 82(14):1204–1211
Lattanzi S, Danni M, Cerqua R, Taffi R, Provinciali L, Silvestrini M (2015) Prediction of disability progression in fingolimod-treated patients. J Neurol Sci 358(1–2):432–434
Prosperini L, Sacca F, Cordioli C et al (2017) Real-world effectiveness of natalizumab and fingolimod compared with self-injectable drugs in non-responders and in treatment-naive patients with multiple sclerosis. J Neurol 264(2):284–294
Rasenack M, Rychen J, Andelova M et al (2016) Efficacy and safety of fingolimod in an unselected patient population. PLoS One 11(1):e0146190
Izquierdo G, Damas F, Paramo MD, Ruiz-Pena JL, Navarro G (2017) The real-world effectiveness and safety of fingolimod in relapsing-remitting multiple sclerosis patients: an observational study. PLoS One 12(4):e0176174
Cohen M, Lebrun C (2014) Moving to fingolimod from natalizumab in multiple sclerosis-reply. JAMA Neurol 71(7):925
Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL (2015) Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 72(2):152–158
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Bevan CJ, Cree BA (2014) Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol 71(3):269–270
Papeix C, Vukusic S, Casey R et al (2016) Risk of relapse after natalizumab withdrawal: results from the French TYSEDMUS cohort. Neurol Neuroimmunol Neuroinflamm 3(6):e297
O’Connor PW, Goodman A, Kappos L et al (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865
Totaro R, Di Carmine C, Costantino G et al (2015) Fingolimod treatment in relapsing-remitting multiple sclerosis patients: a prospective observational multicenter postmarketing study. Mult Scler Int 2015:763418
Yamout BI, Zeineddine MM, Tamim H, Khoury SJ (2015) Safety and efficacy of fingolimod in clinical practice: the experience of an academic center in the Middle East. J Neuroimmunol 289:93–97
Sangalli F, Moiola L, Ferre L et al (2014) Long-term management of natalizumab discontinuation in a large monocentric cohort of multiple sclerosis patients. Mult Scler Relat Disord 3(4):520–526
Horakova D, Kalincik T, Dolezal O et al (2012) Early predictors of non-response to interferon in multiple sclerosis. Acta Neurol Scand 126(6):390–397
Rio J, Castillo J, Rovira A et al (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 15(7):848–853
Romeo M, Martinelli-Boneschi F, Rodegher M et al (2013) Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing-remitting multiple sclerosis patients. Eur J Neurol 20(7):1060–1067
Sormani MP, Rio J, Tintore M et al (2013) Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 19(5):605–612
Sormani MP, Gasperini C, Romeo M et al (2016) Assessing response to interferon-beta in a multicenter dataset of patients with MS. Neurology 87(2):134–140
Acknowledgements
The study was supported by the “Fondazione Italiana Sclerosi Multipla”, project 2013/R/13.
Author information
Authors and Affiliations
Contributions
F. Esposito received honoraria from TEVA and Merck; L. Ferrè reports no disclosures; F. Clarelli reports no disclosures; M. A. Rocca received speakers honoraria from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva and Merk Serono and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla; G. Sferruzza reports no disclosures; L. Storelli reports no disclosures; M. Radaelli reports no disclosures; F. Sangalli reports no disclosures; L. Moiola received honoraria for speaking at meetings or for attending to advisory board from Sanofi-Genzyme, Biogen-Idec, Novartis and TEVA; B. Colombo received reimbursement for travel expenses from Biogen and Genzyme; F. Martinelli Boneschi has received compensation for activities with Teva Neuroscience, Biogen Idec, Merck Serono as speaker and/or advisor; G. Comi has received compensation for consulting services with the following companies: Novartis, Teva, Sanofi, Genzyme, Merck, Biogen, Excemed, Roche, Almirall, Chugai, Receptos, Forward Pharma and compensation for speaking activities from Novartis, Teva, Sanofi, Genzyme, Merk, Biogen, Excemed, Roche; M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on a scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Biogen Idec, ExceMED, Novartis, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer’s Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and ARiSLA (Fondazione Italiana di Ricerca per la SLA); V. Martinelli has received honoraria for consulting and speaking activities from Biogen Merck, Bayer, TEVA, Novartis and Genzyme.
Corresponding author
Additional information
F. Esposito and L. Ferrè contributed equally to the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2018_8791_MOESM1_ESM.tif
Supplementary Fig. 1 Time to FTY discontinuation stratified according to the reasons for drug withdrawal. Survival curves showing the time to discontinuation of FTY stratified according to reasons for drug withdrawal: safety, inefficacy, patient choice (including pregnancy planning) and transfer to another centre. The survival plots are reported for NO_NTZ (A) and NTZ (B) patients, respectively (TIFF 242 kb)
Rights and permissions
About this article
Cite this article
Esposito, F., Ferrè, L., Clarelli, F. et al. Effectiveness and baseline factors associated to fingolimod response in a real-world study on multiple sclerosis patients. J Neurol 265, 896–905 (2018). https://doi.org/10.1007/s00415-018-8791-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8791-1