Abstract
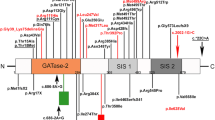
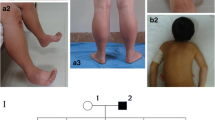
We investigated three unrelated patients with tubular-aggregate myopathy and slowly progressive muscle weakness manifesting in the first years of life. All patients showed type 1 muscle fiber predominance and hypotrophy of type 2 fibers. Tubular aggregates were abundant. In all three patients mutations were identified in the gene STIM1, and the mutations were found to be de novo in all patients. In one of the patients the mutation was identified by exome sequencing. Two patients harbored the previously described mutation c.326A>G p.(His109Arg), while the third patient had a novel mutation c.343A>T p.(Ile115Phe). Taking our series together with previously published cases, the c.326A>G p.(His109Arg) seems to be a hotspot mutation that is characteristically related to early onset muscle weakness.



Similar content being viewed by others
References
Engel WK (1964) Mitochondrial aggregates in muscle disease. J Histochem Cytochem: Off J Histochem Soc 12:46–48
Schiaffino S (2012) Tubular aggregates in skeletal muscle: just a special type of protein aggregates? Neuromuscul Disord 22:199–207. doi:10.1016/j.nmd.2011.10.005
Funk F, Ceuterick-de Groote C, Martin JJ et al (2013) Morphological spectrum and clinical features of myopathies with tubular aggregates. Histol Histopathol 28:1041–1054
Bohm J, Chevessier F, Maues De Paula A et al (2013) Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet 92:271–278. doi:10.1016/j.ajhg.2012.12.007
Tulinius MH, Lundberg A, Oldfors A (1996) Early-onset myopathy with tubular aggregates. Pediatr Neurol 15:68–71
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. doi:10.1093/bioinformatics/btp324
McKenna A, Hanna M, Banks E et al (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi:10.1101/gr.107524.110
DePristo MA, Banks E, Poplin R et al (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498. doi:10.1038/ng.806
Cingolani P, Platts A, le Wang L et al (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6:80–92. doi:10.4161/fly.19695
Putney JW Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12
Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85:757–810. doi:10.1152/physrev.00057.2003
Field ML, Khan O, Abbaraju J, Clark JF (2006) Functional compartmentation of glycogen phosphorylase with creatine kinase and Ca2+ ATPase in skeletal muscle. J Theor Biol 238:257–268. doi:10.1016/j.jtbi.2005.05.017
Wanson JC, Drochmans P (1972) Role of the sarcoplasmic reticulum in glycogen metabolism. Binding of phosphorylase, phosphorylase kinase, and primer complexes to the sarcovesicles of rabbit skeletal muscle. J Cell Biol 54:206–224
Stephenson DG (2011) In pursuit of the glycogen–[Ca2+] connection. J Physiol 589:451. doi:10.1113/jphysiol.2010.203943
Stoward PJ, Pearse AGE (eds) (1991) Histochemistry: theoretical and applied. Enzyme histochemistry, 4th edn, vol 3. Churchill-Livingstone, Edinburgh, xiv + pp1-727
Zheng L, Stathopulos PB, Schindl R, Li GY, Romanin C, Ikura M (2011) Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci USA 108:1337–1342. doi:10.1073/pnas.1015125108
Liou J, Kim ML, Heo WD et al (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol: CB 15:1235–1241. doi:10.1016/j.cub.2005.05.055
Roos J, DiGregorio PJ, Yeromin AV et al (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169:435–445. doi:10.1083/jcb.200502019
Zhang SL, Yu Y, Roos J et al (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437:902–905. doi:10.1038/nature04147
Kiviluoto S, Decuypere JP, De Smedt H, Missiaen L, Parys JB, Bultynck G (2011) STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet Muscle 1:16. doi:10.1186/2044-5040-1-16
Acknowledgments
Funding was provided from the Italian Ministry of Health Ricerca Finalizzata (to EB, AD, SP, GT and FF), the Italian Ministry of Health Ricerca Corrente (to MN, AC and MT), and the Swedish Research Council (to AO; Proj. No 7122).
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Tartaglia, A. Oldfors, and E. Bertini contributed equally as the senior investigators in this project.
Rights and permissions
About this article
Cite this article
Hedberg, C., Niceta, M., Fattori, F. et al. Childhood onset tubular aggregate myopathy associated with de novo STIM1 mutations. J Neurol 261, 870–876 (2014). https://doi.org/10.1007/s00415-014-7287-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7287-x