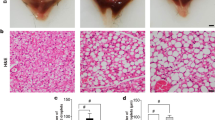
Abstract
Diagnosis of fatal hypothermia is considered to be difficult in forensic practice and even if findings due to cold exposure are evident, cold exposure is not necessarily a direct cause of death. Identification of useful molecular markers for the diagnosis of fatal hypothermia has not been successful. In this study, to identify novel molecular markers that inform the diagnosis of fatal hypothermia, we focused on skeletal muscle, which plays a role in cold-induced thermogenesis in mammals. We made rat models of mild, moderate, and severe hypothermia and performed body temperature-dependent gene expression analysis in the iliopsoas muscle using next-generation sequencing (NGS). NGS showed that after severe hypothermia, the expression levels of 91 mRNAs were more than double those in mild and moderate hypothermia and control animals. Gene ontology (GO) analysis indicated that these mRNAs are involved in a number of biological processes, including response to stress and lipids, and cellular response to hypoxia. The expression of four genes [connective tissue growth factor (Ctgf), JunB proto-oncogene, AP-1 transcription factor subunit (Junb), nuclear receptor subfamily 4, group A, member 1 (Nr4a1), and Syndecan 4 (Sdc4)] and the level of one protein (CTGF) were induced only by severe hypothermia. These genes and protein are involved in muscle regeneration, tissue repair, and lipid metabolism. These results indicate that heat production to maintain body temperature in a process leading to fatal hypothermia might be performed by the iliopsoas muscle, and that Ctgf, Junb, Nr4a1, and Sdc4 genes are potential diagnostic markers for fatal hypothermia.







Similar content being viewed by others
References
Hirvonen J (1976) Necropsy findings in fatal hypothermia cases. Forensic Sci 8:155–164
Mizukami H, Shimizu K, Shiono H, Uezono T, Sasaki M (1999) Forensic diagnosis of death from cold. Legal Med 1:204–209
Turk EE (2010) Hypothermia. Forensic Sci Med Pathol 6:106–115
Brändström H, Eriksson A, Giesbrecht G, Angquist KA, Haney M (2012) Fatal hypothermia: an analysis from a sub-arctic region. Int J Circumpolar Health 71:1–7
Ogata M, Ago K, Ago M, Kondo T, Kasai K, Ishikawa T, Mizukami H (2007) A fatal case of hypothermia associated with hemorrhages of the pectoralis minor, intercostal, and iliopsoas muscles. Am J Forensic Med Pathol 28:348–352
Preuss J, Lignitz E, Dettmeyer R, Madea B (2007) Pancreatic changes in cases of death due to hypothermia. Forensic Sci Int 166:194–198
Hejna P, Zátopková L, Tsokos M (2012) The diagnostic value of synovial membrane hemorrhage and bloody discoloration of synovial fluid (“inner knee sign”) in autopsy cases of fatal hypothermia. Int J Legal Med 126:415–419
Ishikawa T, Yoshida C, Michiue T, Perdekamp MG, Pollak S, Maeda H (2010) Immunohistochemistry of catecholamines in the hypothalamic-pituitary-adrenal system with special regard to fatal hypothermia and hyperthermia. Legal Med 12:121–127
Kitamura O, Gotohda T, Ishigami A, Tokunaga I, Kubo S, Nakasono I (2005) Effect of hypothermia on postmortem alterations in MAP2 immunostaining in the human hippocampus. Legal Med 7:340–344
Umehara T, Usumoto Y, Tsuji A, Kudo K, Ikeda N (2011) Expression of material mRNA in the hypothalamus and frontal cortex in a rat model of fatal hypothermia. Legal Med 13:165–170
Takamiya M, Saigusa K, Dewa K (2013) DNA microarray analysis of the mouse adrenal gland for the detection of hypothermia biomarkers: potential usefulness for forensic investigation. Ther Hypothermia Temp Manag 3:63–73
Hirvonen J, Penttinen J, Huttunen P, Saukko P (1980) Changes in the myocardium and skeletal muscle in Guinea pigs in cold exposure with and without ethanol. Z Rechtsmed 84:195–207
Sadler DW, Pounder DJ (1995) Urinary catecholamines as markers of hypothermia. Forensic Sci Int 76:227–230
Hirvonen J, Huttunen P (1995) Hypothermia markers: serum, urine and adrenal gland catecholamines in hypothermic rats given ethanol. Forensic Sci Int 72:125–133
Ishikawa T, Miyaishi S, Tachibana T, Ishizu H, Zhu BL, Maeda H (2004) Fatal hypothermia related vacuolation of hormone-producing cells in the anterior pituitary. Legal Med 6:157–163
Rada A, Tonino P, Anselmi G, Strauss M (2005) Is hypothermia a stress condition in HepG2 cells? Expression and localization of Hsp70 in human hepatoma cell line. Tissue Cell 37:59–65
Zhu BL, Ishikawa T, Michiue T, Li DR, Zhao D, Quan L, Oritani S, Bessho Y, Maeda H (2007) Postmortem serum catecholamine levels in relation to the cause of death. Forensic Sci Int 173:122–129
Maeda H, Zhu BL, Bessho Y, Ishikawa T, Quan L, Michiue T, Zhao D, Li DR, Komatsu A (2008) Postmortem serum nitrogen compounds and C-reactive protein levels with special regard to investigation of fatal hyperthermia. Forensic Sci Med Pathol 4:175–180
Preuss J, Dettmeyer R, Poster S, Lignitz E, Madea B (2008) The expression of heat shock protein 70 in kidneys in cases of death due to hypothermia. Forensic Sci Int 176:248–252
Ishikawa T, Quan L, Li DR, Zhao D, Michiue T, Hamel M, Maeda H (2008) Postmortem biochemistry and immunohistochemistry of adrenocorticotropic hormone with special regard to fatal hypothermia. Forensic Sci Int 179:147–151
Li DR, Michiue T, Zhu BL, Ishikawa T, Quan L, Zhao D, Yoshida C, Chen JH, Wang Q, Komatsu A, Azuma Y, Maeda H (2009) Evaluation of postmortem S100B levels in the cerebrospinal fluid with regard to the cause of death in medicolegal autopsy. Legal Med 11(Suppl 1):S273–S275
Jakubeniene M, Chaker GA, Becelis A, Malakiene D, Raudys R (2009) Investigation of calcium and sodium in postmortem material as biochemical markers defining the cause of death from hypothermia. Legal Med 11(Suppl 1):S304–S306
Ishikawa T, Michiue T, Zhao D, Komatsu A, Azuma Y, Quan L, Hamel M, Maeda H (2009) Evaluation of postmortem serum and cerebrospinal fluid levels of thyroid-stimulating hormone with special regard to fatal hypothermia. Legal Med 11(Suppl 1):S228–S230
Zhou C, Byard RW (2011) Armanni-Ebstein phenomenon and hypothermia. Forensic Sci Int 206:e82–e84
Yoshida C, Ishikawa T, Michiue T, Quan L, Maeda H (2011) Postmortem biochemistry and immunohistochemistry of chromogranin A as a stress marker with special regard to fatal hypothermia and hyperthermia. Int J Legal Med 125:11–20
Palmiere C, Sporkert F, Werner D, Bardy D, Augsburger M, Mangin P (2012) Blood, urine and vitreous isopropyl alcohol as biochemical markers in forensic investigations. Legal Med 14:17–20
Palmiere C, Lesta MM, Sabatasso S, Mangin P, Augsburger M, Sporkert F (2012) Usefulness of postmortem biochemistry in forensic pathology: illustrative case reports. Legal Med 14:27–35
Palmiere C, Bardy D, Letovanec I, Mangin P, Augsburger M, Ventura F, Iglesias K, Werner D (2013) Biochemical markers of fatal hypothermia. Forensic Sci Int 226:54–61
Quan L, Ishikawa T, Michiue T, Li DR, Zhao D, Zhu BL, Maeda H (2005) Quantitative analysis of ubiquitin-immunoreactivity in the midbrain periaqueductal gray matter with regard to the causes of death in forensic autopsy. Legal Med 7:151–156
Doberentz E, Preuss-Wössner J, Kuchelmeister K, Madea B (2011) Histological examination of the pituitary glands in cases of fatal hypothermia. Forensic Sci Int 207:46–49
Park JJ, Lee HK, Shin MW, Kim SJ, Noh SY, Shin J, Yu WS (2007) Short-term cold exposure may cause a local decrease of neuropeptide Y in the rat hypothalamus. Mol Cells 23:88–93
Jedema HP, Gold SJ, Gonzalez-Burgos G, Sved AF, Tobe BJ, Wensel T, Grace AA (2008) Chronic cold exposure increases RGS7 expression and decreases alpha(2)-autoreceptor-mediated inhibition of noradrenergic locus coeruleus neurons. Eur J Neurosci 27:2433–2443
Sánchez E, Uribe RM, Corkidi G, Zoeller RT, Cisneros M, Zacarias M, Morales-Chapa C, Charli JL, Joseph-Bravo P (2001) Differential responses of thyrotropin-releasing hormone (TRH) neurons to cold exposure or suckling indicate functional heterogeneity of the TRH system in the paraventricular nucleus of the rat hypothalamus. Neuroendocrinology 74:407–422
Mollica MP, Lionetti L, Crescenzo R, Tasso R, Barletta A, Liverini G, Iossa S (2005) Cold exposure differently influences mitochondrial energy efficiency in rat liver and skeletal muscle. FEBS Lett 579:1978–1982
Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD (2008) Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One 3:e1777
Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122:545–552
Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H (2003) Proposal for a unified CCN nomenclature. Mol Pathol 56:127–128
Vial C, Zúñiga LM, Cabello-Verrugio C, Cañón P, Fadic R, Brandan E (2008) Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol 215:410–421
Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E (2012) Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med 16:752–764
Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E (2011) CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol 225:490–501
Brigstock DR (2002) Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 5:153–165
Nishida T, Kubota S, Aoyama E, Janune D, Lyons KM, Takigawa M (2015) CCN family protein 2 (CCN2) promotes the early differentiation, but inhibits the terminal differentiation of skeletal myoblasts. J Biochem 157:91–100
Morales MG, Acuña MJ, Cabrera D, Goldschmeding R, Brandan E (2018) The pro-fibrotic connective tissue growth factor (CTGF/CCN2) correlates with the number of necrotic-regenerative foci in dystrophic muscle. J Cell Commun Signal 12:413–421
Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA (2013) Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12:75–87
Le Grand F, Jones AE, Seale V, Scimè A, Rudnicki MA (2009) Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4:535–547
Pinent M, Prokesch A, Hackl H, Voshol PJ, Klatzer A, Walenta E, Panzenboeck U, Kenner L, Trajanoski Z, Hoefler G, Bogner-Strauss JG (2011) Adipose triglyceride lipase and hormone-sensitive lipase are involved in fat loss in JunB-deficient mice. Endocrinology 152:2678–2689
Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE (2005) Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem 280:12573–12584
Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P (2006) NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12:1048–1055
Fassett MS, Jiang W, D'Alise AM, Mathis D, Benoist C (2012) Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci 109:3891–3896
Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 12:778–785
Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF (2006) Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med 203:719–729
Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H, de Vries CJ (2002) Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation 106:1530–1535
Cortez-Toledo O, Schnair C, Sangngern P, Metzger D, Chao LC (2017) Nur77 deletion impairs muscle growth during developmental myogenesis and muscle regeneration in mice. PLoS One 12:e0171268
Acknowledgements
We thank Jeremy Allen, PhD, from Edanz Group (www.edanzediting.com/ac) for editing the draft of this manuscript.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research C, 16K09210).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Animal Care Committee of Nagasaki University approved this research protocol (approval number 1606081312).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure. 1
In the severe hypothermia group, the expression levels of 77 mRNAs were less than half those in control, mild and moderate hypothermia groups. The heatmap illustrates profiles of 77 mRNAs from the iliopsoas muscle of control, mild, moderate and severe hypothermia rats. Quantification and log2 calculations were performed using Deseq, and normalization for each gene was performed using the median of all samples. (PNG 680 kb)
Figure. 2
A. In the moderate hypothermia group, 67 mRNAs showed a more than twofold difference in expression level compared with levels in control, mild and severe hypothermia groups. B. In the mild hypothermia group, 28 mRNAs showed a more than twofold difference in expression level compared with levels in control, moderate and severe hypothermia groups. The heatmap illustrates profiles of 67 and 28 mRNAs from the iliopsoas muscle of control, mild, moderate and severe hypothermia rats. Quantification and log2 calculations were performed using Deseq, and normalization for each gene was performed using the median of all samples. (PNG 826 kb)
Rights and permissions
About this article
Cite this article
Umehara, T., Murase, T., Abe, Y. et al. Identification of potential markers of fatal hypothermia by a body temperature-dependent gene expression assay. Int J Legal Med 133, 335–345 (2019). https://doi.org/10.1007/s00414-018-1888-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-018-1888-3