Abstract
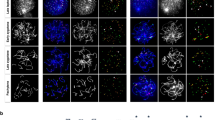
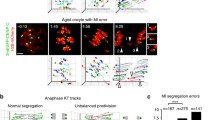
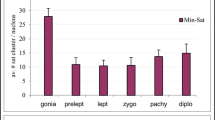
In mammalian oocytes, proper chromosome segregation at the first meiotic division is dictated by the presence and site of homologous chromosome recombination, which takes place in fetal life. Our current understanding of how homologous chromosomes find each other and initiate synapsis, which is prerequisite for homologous recombination, is limited. It is known that chromosome telomeres are anchored into the nuclear envelope (NE) at the early meiotic prophase I (MPI) and move along NE to facilitate homologous chromosome search and pairing. However, the mouse (Mus musculus) carries all acrocentric chromosomes with one telomeric end close to the centromere (subcentromeric telomere; C-telomere) and the other far away from the centromere (distal telomere; D-telomere), and how C- and D-telomeres participate in chromosome pairing and synapsis during the MPI progression is not well understood. Here, we found in the mouse oocyte that C- and D-telomeres transiently clustered in one area, but D-telomeres soon separated together from C-telomeres and then dispersed to preferentially initiate synapsis, while C-telomeres remained in clusters and synapsed at the last. In the Spo11 null oocyte, which is deficient in SPO11-dependent DSBs formation and homologous synapsis, the pattern of C- and D-telomere clustering and resolution was not affected, but synapsis was more frequently initiated at C-telomeres. These results suggest that SPO11 suppresses the early synapsis between C-telomeres in clusters.








Similar content being viewed by others
References
Andrey P, Kiêu K, Kress C, Lehmann G, Tirichine L, Liu Z, Biot E, Adenot P-G, Hue-Beauvais C, Houba-Hérin N (2010) Statistical analysis of 3D images detects regular spatial distributions of centromeres and chromocenters in animal and plant nuclei. PLoS Com Biol 6:e1000853
Ashley T, Plug AW, Xu J, Solari AJ, Reddy G, Golub EI, Ward DC (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104:19–28
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6:989–998
Boateng KA, Bellani MA, Gregoretti IV, Pratto F, Camerini-Otero RD (2013) Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev Cell 24:196–205
Bolcun-Filas E, Costa Y, Speed R, Taggard M, Benavente R, De Rooij DG (2007) SYCE2 is required for synaptonemal complex assembly, double strand break repair, and homologous recombination. J Cell Biol 176:741–747
Brick K, Thibault-Sennett S, Smagulova F, Lam KW, Pu Y, Pratto F, Camerini-Otero RD, Petukhova GV (2018) Extensive sex differences at the initiation of genetic recombination. Nature 561:338–342
Cannavo E, Sanchez A, Anand R, Ranjha L, Hugener J, Adam C, Acharya A, Weyland N, Aran-Guiu X, Charbonnier J-B, Hoffmann ER, Borde V, Matos J, Cejka P (2020) Regulation of the MLH1-MLH3 endonuclease in meiosis. Nature 586:618–622
Canudas S, Smith S (2009) Differential regulation of telomere and centromere cohesion by the Scc3 homologues SA1 and SA2, respectively, in human cells. J Cell Biol 187:165–173
Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y (2006) Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125:59–69
Cole F, Kauppi L, Lange J, Roig I, Wang R, Keeney S, Jasin M (2012) Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat Cell Biol 14:424–430
de Massy B (2013) Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu Rev Genet 47:563–599
Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M (2007) SUN1 is required for telomre attachment to nuclear envelope and gametogenesis in mice. Dev Cell 12:863–872
Garagna S, Page J, Fernandez-Donoso R, Zuccotti M, Searle JB (2014) The Robertsonian phenomenon in the house mouse: mutation, meiosis and speciation. Chromosoma 123:529–544
Gerton JL, Hawley RS (2005) Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev Genet 6:477–487
Gropp A, Winking H, Zech L, Müller H (1972) Robertsonian chromosomal variation and identification of metacentric chromosomes in feral mice. Chromosoma 39:265–288
Gruhn JR, Al-Asmar N, Fasnacht R, Maylor-Hagen H, Peinado V, Rubio C, Broman KW, Hunt PA, Hassold T (2016) Correlations between synaptic initiation and meiotic recombination: a study of humans and mice. Am J Hum Genet 98:102–115
Gruhn JR, Zielinska AP, Shukla V, Blanshard R, Capalbo A, Cimadomo D, Nikiforov D, Chan AC-H, Newnham LJ, Vogel I (2019) Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 365:1466–1469
Guillon H, de Massy B (2002) An initiation site for meiotic crossing-over and gene conversion in the mouse. Nat Genet 32:296–299
Guillon H, Baudat F, Grey C, Liskay RM, de Massy B (2005) Crossover and noncrossover pathways in mouse meiosis. Mol Cell 20:563–573
Guiraldelli MF, Eyster C, Wilkerson JL, Dresser ME, Pezza RJ (2013) Mouse HFM1/Mer3 is required for crossover formation and complete synapsis of homologous chromosomes during meiosis. PLoS Genet 9:e1003383
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291
Klutstein M, Fennell A, Fernández-Álvarez A, Cooper JP (2015) The telomere bouquet regulates meiotic centromere assembly. Nat Cell Biol 17:458–469
Koehler KE, Millie EA, Cherry JP, Burgoyne PS, Evens EP, Hunt PA, Hassold TJ (2002) Sex-specific differences in meiotic chromosome segregation revealed by dicentric bridge resolution in mice. Genetics 162:1367–1379
Koszul R, Kleckner N (2009) Dynamic chromosome movements during meiosis: a way to eliminate unwanted connections? Trends Cell Biol 19:716–724
Lee C-Y, Horn HF, Stewart CL, Burke B, Bolcun-Filas E, Schimenti JC, Dresser ME, Pezza RJ (2015) Mechanism and regulation of rapid telomere prophase movements in mouse meiotic chromosomes. Cell Rep 11:551–563
Liebe B, Alsheimer M, Höög C, Benavente R, Scherthan H (2004) Telomere attachment, meiotic chromosome condensation, pairing, and bouquet stage duration are modified in spermatocytes lacking axial elements. Mol Biol Cell 15:827–837
Martinez-Perez E, Colaiacova MP (2009) Distribution of meiotic recombination events: talking to your neighbors. Curr Op Genet Dev 19:105–112
Moens PB, Heyting C, Dietrich AJJ, van Raamsdonk W, Chen Q (1987) Synaptonemal complex antigen location and conservation. J Cell Biol 105:93–103
Moens PB, Marcon E, Shore JS, Kochakpour N, Spyropoulos B (2007) Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. J Cell Sci 120:1017–1027
Morimoto A, Shibuya H, Zhu X, Kim J, K-i I, Han M, Watanabe Y (2012) A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol 198:165–172
Neale MJ, Keeney S (2006) Clarifying the mechanics of DNA strand exchange in meiotic recombination. Nature 442:153–158
Ottolini CS, Newnham LJ, Capalbo A, Natesan SA, Joshi HA, Cimadomo D, Griffin DK, Sage K, Summers MC, Thornhill AR (2015) Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat Genet 47:727–735
Pawlowski WP, Cande WZ (2005) Coordinating the events of the meiotic prophase. Trends Cell Biol 15:674–681
Remeseiro S, Cuadrado A, Carretero M, Martínez P, Drosopoulos WC, Cañamero M, Schildkraut CL, Blasco MA, Losada A (2012) Cohesin-SA1 deficiency drives aneuploidy and tumourigenesis in mice due to impaired replication of telomeres. EMBO J 31:2076–2089
Reynolds A, Qiao H, Yang Y, Chen JK, Jackson N, Biswas K, Holloway JK, Baudat F, de Massy B, Wang J (2013) RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis. Nat Genet 45:269–278
Rockmill B, Roeder GS (1998) Telomere-mediated chromosome pairing during meiosis in budding yeast. Genes Dev 12:2574–2586
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Saferali A, Berlivet S, Schimenti J, Bartolomei MS, Taketo T, Naumova AK (2010) Defective imprint resetting in carriers of Robertsonian translocation Rb (8.12). Mamm Genome 21:377–387
Scherthan H (2007) Telomere attachment and clustering during meiosis. Cell Mol Life Sci 64:117–124
Scherthan H, Weich S, Schwegler H, Heyting C, Härle M, Cremer T (1996) Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 134:1109–1125
Shibuya H, Watanabe Y (2014) The meiosis-specific modification of mammalian telomeres. Cell Cycle 13:2024–2028
Shibuya H, Ishiguro K-i, Watanabe Y (2014a) The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat Cell Biol 16:145–156
Shibuya H, Morimoto A, Watanabe Y (2014b) The dissection of meiotic chromosome movement in mice using an in vivo electroporation technique. PLoS Genet 10:e1004821
Shibuya H, Hernández-Hernández A, Morimoto A, Negishi L, Höög C, Watanabe Y (2015) MAJIN links telomeric DNA to the nuclear membrane by exchanging telomere cap. Cell 163:1252–1266
Taketo T (2012) Microspread oocyte preparations for the analysis of meiotic prophase progression with improved recovery by cytospin centrifugation. Methods Mol Biol 825:173–181
Tankimanova M, Hulten MA, Tease C (2004) The initiation of homologous chromosome synapsis in mouse fetal oocytes is not directly driven by centromere and telomere clustering in the bouquet. Cytogenet Genome Res 105:172–181
Trelles-Sticken E, Loidl J, Scherthan H (1999) Bouquet formation in budding yeast: initiation of recombination is not required for meiotic telomere clustering. J Cell Sci 112:651–658
Trelles-Sticken E, Dresser ME, Scherthan H (2000) Meiotic telomere protein Ndj1p is required for meiosis-specific telomere distribution, bouquet formation and efficient homologue pairing. J Cell Biol 151:95–106
Wallace BMN, Searle JB, Everett CA (1992) Male meiosis and gametogenesis in wild house mice (Mus musculus domesticus) from a chromosomal hybrid zone; a comparison between "simple" Robertsonian heterozygotes and homozygotes. Cytogenet Cell Genet 61:211–220
Yuan L, Liu J-G, Hoja M-R, Wilbertz J, Nordqvist K, Höög C (2002) Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296:1115–1118
Acknowledgments
We are grateful to Dr. Thomas Boudier (Université Pierre et Marie Curie, France) for his advice on telomere clustering analyses.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was partially supported by the grants from the Natural Sciences and Engineering Research Council of Canada (NSERC 77914) and Canadian Institutes of Health Research (CIHR, MOP-137028) to TT. PK received the McGill Center for Research in Reproduction and Development (CRRD) studentship.
Author information
Authors and Affiliations
Contributions
T.T. directed the study. T.T. and P.K. contributed the design and P.K. performed the experiments. T.T. and P.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Kazemi, P., Taketo, T. Two telomeric ends of acrocentric chromosome play distinct roles in homologous chromosome synapsis in the fetal mouse oocyte. Chromosoma 130, 41–52 (2021). https://doi.org/10.1007/s00412-021-00752-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-021-00752-1