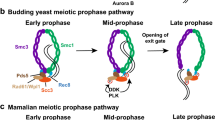
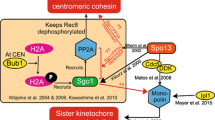
Abstract
Sister chromatid cohesion is regulated by cohesin complexes and topoisomerase IIα. Although relevant studies have shed some light on the relationship between these two mechanisms of cohesion during mammalian mitosis, their interplay during mammalian meiosis remains unknown. In the present study, we have studied the dynamics of topoisomerase IIα in relation to that of the cohesin subunits RAD21 and REC8, the shugoshin-like 2 (Schizosaccharomyces pombe) (SGOL2) and the polo-like kinase 1-interacting checkpoint helicase (PICH), during both male mouse meiotic divisions. Our results strikingly show that topoisomerase IIα appears at stretched strands connecting the sister kinetochores of segregating early anaphase II chromatids, once the cohesin complexes have been removed from the centromeres. Moreover, the number and length of these topoisomerase IIα-connecting strands increase between lagging chromatids at anaphase II after the chemical inhibition of the enzymatic activity of topoisomerase IIα by etoposide. Our results also show that the etoposide-induced inhibition of topoisomerase IIα is not able to rescue the loss of centromere cohesion promoted by the absence of the shugoshin SGOL2 during anaphase I. Taking into account our results, we propose a two-step model for the sequential release of centromeric cohesion during male mammalian meiosis II. We suggest that the cohesin removal is a prerequisite for the posterior topoisomerase IIα-mediated resolution of persisting catenations between segregating chromatids during anaphase II.









Similar content being viewed by others
References
Adelman CA, Petrini JH (2008) ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. PLoS Genet 4:e1000042
Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6:253–268
Baumann C, Korner R, Hofmann K, Nigg EA (2007) PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128:101–114
Chan KL, North PS, Hickson ID (2007) BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J 26:3397–3409
Cobb J, Miyaike M, Kikuchi A, Handel MA (1999) Meiotic events at the centromeric heterochromatin: histone H3 phosphorylation, topoisomerase IIα localization and chromosome condensation. Chromosoma 108:412–425
Eckley DM, Ainsztein AM, Mackay AM, Goldberg IG, Earnshaw WC (1997) Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and midanaphase cells. J Cell Biol 136:1169–1183
Farcas AM, Uluocak P, Helmhart W, Nasmyth K (2011) Cohesin's concatenation of sister DNAs maintains their intertwining. Mol Cell 44:97–107
Floridia G, Zatterale A, Zuffardi O, Tyler-Smith C (2000) Mapping of a human centromere onto the DNA by topoisomerase II cleavage. EMBO Rep 1:489–493
Gómez R, Valdeolmillos A, Parra MT, Viera A, Carreiro C, Roncal F, Rufas JS, Barbero JL, Suja JA (2007) Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep 8:173–180
Gutiérrez-Caballero C, Herrán Y, Sánchez-Martín M, Suja JA, Barbero JL, Llano E, Pendás AM (2011) Identification and molecular characterization of the mammalian α-kleisin RAD21L. Cell Cycle 10:1477–1487
Gutiérrez-Caballero C, Cebollero LR, Pendás AM (2012) Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet 28:351–360
Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol 3:e69
Herrán Y, Gutiérrez-Caballero C, Sánchez-Martín M, Hernández T, Viera A, Barbero JL, de Álava E, de Rooij DG, Suja JA, Llano E et al (2011) The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 30:3091–3105
Hirano T (2012) Condensins: universal organizers of chromosomes with diverse functions. Genes Dev 26:1659–1678
Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y (2011) A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 12:267–275
Jonstrup AT, Thomsen T, Wang Y, Knudsen BR, Koch J, Andersen AH (2008) Hairpin structures formed by alpha satellite DNA of human centromeres are cleaved by human topoisomerase IIα. Nucleic Acids Res 36:6165–6174
Kallio M, Lähdetie J (1996) Fragmentation of centromeric DNA and prevention of homologous chromosome separation in male mouse meiosis in vivo by the topoisomerase II inhibitor etoposide. Mutagenesis 11:435–443
Kaulich M, Cubizolles F, Nigg EA (2012) On the regulation, function, and localization of the DNA-dependent ATPase PICH. Chromosoma 121:395–408
Ke Y, Huh JW, Warrington R, Li B, Wu N, Leng M, Zhang J, Ball HL, Li B, Yu H (2011) PICH and BLM limit histone association with anaphase centromeric DNA threads and promote their resolution. EMBO J 30:3309–3321
Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441:46–52
Klein F, Laroche T, Cardenas ME, Hofmann JF, Schweizer D, Gasser SM (1992) Localization of RAP1 and topoisomerase II in nuclei and meiotic chromosomes of yeast. J Cell Biol 117:935–948
Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu H, Helmhart W, Kudo H, McKay M, Maro B et al (2006) Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126:135–146
Lee MT, Bachant J (2009) SUMO modification of DNA topoisomerase II: trying to get a CENse of it all. DNA Repair 8:557–568
Lee J, Hirano T (2011) RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol 192:263–276
Lee J, Iwai T, Yokota T, Yamashita M (2003) Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 116:2781–2790
Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10:42–52
Llano E, Gómez R, Gutiérrez-Caballero C, Herrán Y, Sánchez-Martín M, Vázquez-Quinones L, Hernández T, de Álava E, Cuadrado A, Barbero JL et al (2008) Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22:2400–2413
Marchetti F, Bishop JB, Lowe X, Generoso WM, Hozier J, Wyrobek AJ (2001) Etoposide induces heritable chromosomal aberrations and aneuploidy during male meiosis in the mouse. Proc Natl Acad Sci U S A 98:3952–3957
Matulis S, Handel MA (2006) Spermatocyte responses in vitro to induced DNA damage. Mol Reprod Dev 73:1061–1072
McNicoll F, Stevense M, Jessberger R (2013) Cohesin in gametogenesis. Curr Top Dev Biol 102:1–34
Moens PB (1990) Unravelling meiotic chromosomes: topoisomerase II and other proteins. J Cell Sci 97:1–3
Moens PB, Earnshaw WC (1989) Anti-topoisomerase II recognizes meiotic chromosome cores. Chromosoma 98:317–322
Murray AW, Szostak JW (1985) Chromosome segregation in mitosis and meiosis. Annu Rev Cell Biol 1:289–315
Nasmyth K (2011) Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13:1170–1177
Nitiss JL (2009a) DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer 9:327–337
Nitiss JL (2009b) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9:338–350
Oliveira RA, Hamilton RS, Pauli A, Davis I, Nasmyth K (2010) Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat Cell Biol 12:185–192
Page J, Suja JA, Santos JL, Rufas JS (1998) Squash procedure for protein immunolocalization in meiotic cells. Chromosom Res 6:639–642
Parra MT, Page J, Yen TJ, He D, Valdeolmillos A, Rufas JS, Suja JA (2002) Expression and behaviour of CENP-E at kinetochores during mouse spermatogenesis. Chromosoma 111:53–61
Parra MT, Viera A, Gómez R, Page J, Carmena M, Earnshaw WC, Rufas JS, Suja JA (2003) Dynamic relocalization of the chromosomal passenger complex proteins inner centromere protein (INCENP) and aurora-B kinase during male mouse meiosis. J Cell Sci 116:961–974
Parra MT, Viera A, Gómez R, Page J, Benavente R, Santos JL, Rufas JS, Suja JA (2004) Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci 117:1221–1234
Parra MT, Gómez R, Viera A, Page J, Calvente A, Wordeman L, Rufas JS, Suja JA (2006) A perikinetochoric ring defined by MCAK and Aurora-B as a novel centromere domain. PLoS Genet 2:e84
Peters AH, Plug AW, van Vugt MJ, de Boer P (1997) A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosom Res 5:66–68
Porter AC, Farr CJ (2004) Topoisomerase II: untangling its contribution at the centromere. Chromosom Res 12:569–583
Prieto I, Suja JA, Pezzi N, Kremer L, Martínez-A C, Rufas JS, Barbero JL (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3:761–766
Prieto I, Pezzi N, Buesa JM, Kremer L, Barthelemy I, Carreiro C, Roncal F, Martínez A, Gómez L, Fernández R et al (2002) STAG2 and Rad21 mammalian mitotic cohesins are implicated in meiosis. EMBO Rep 3:543–550
Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Gálová M, Petronczki M, Gregan J, Cetin B et al (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441:53–61
Russell LB, Hunsicker PR, Kerley M, Pyle A, Saxton AM (2004) Etoposide exposure during male mouse pachytene has complex effects on crossing-over and causes nondisjunction. Mutat Res 565:61–77
Spence JM, Critcher R, Ebersole TA, Valdivia MM, Earnshaw WC, Fukagawa T, Farr CJ (2002) Co-localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha-satellite array. EMBO J 21:5269–5280
Spence JM, Phua HH, Mills W, Carpenter AJ, Porter AC, Farr CJ (2007) Depletion of topoisomerase IIα leads to shortening of the metaphase interkinetochore distance and abnormal persistence of PICH-coated anaphase threads. J Cell Sci 120:3952–3964
Suja JA, Barbero JL (2009) Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn 5:94–116
Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K (2010) Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 24:2505–2516
Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell 10:575–585
Toyoda Y, Yanagida M (2006) Coordinated requirements of human topo II and cohesin for metaphase centromere alignment under Mad2-dependent spindle checkpoint surveillance. Mol Biol Cell 17:2287–2302
Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400:37–42
Vagnarelli P, Morrison C, Dodson H, Sonoda E, Takeda S, Earnshaw WC (2004) Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep 5:167–171
Viera A, Gómez R, Parra MT, Schmiesing JA, Yokomori K, Rufas JS, Suja JA (2007) Condensin I reveals new insights on mouse meiotic chromosome structure and dynamics. PLoS ONE 2:e783
Wang LH, Schwarzbraun T, Speicher MR, Nigg EA (2008) Persistence of DNA threads in human anaphase cells suggests late completion of sister chromatid decatenation. Chromosoma 117:123–135
Wang LH, Mayer B, Stemmann O, Nigg EA (2010) Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J Cell Sci 123:806–813
Wolf KW, Baumgart K, Winking H (1988) Meiotic association and segregation of the achiasmatic giant sex chromosomes in the male field vole (Microtus agrestis). Chromosoma 97:124–133
Acknowledgments
We thank Linda Wordeman, Bill Earnshaw, Jibak Lee and Erich A. Nigg for providing MCAK, INCENP, REC8 and PICH antibodies, respectively; Lorena Barreras for her technical assistance; and Kim Nasmyth for providing REC8-myc transgenic male mice. This work was supported by grants BFU2009-10987/BCM to A.V., BFU2009-08975 to J.L.B., SAF2011-25252 to A.M.P. and SAF2011-28842-C02-01 to J.A.S. from the Ministerio de Ciencia e Innovación and Ministerio de Economía y Competitividad (Spain) and grant FMM AP98712012 from the Fundación Mutua Madrileña to J.L.B.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Distribution of Topo IIα and SYCP3 in metaphase I and anaphase I chromosomes. Double immunolabelling of Topo IIα (green) and SYCP3 (red) in selected metaphase I bivalents (a, b) and an anaphase I chromosome (c) from spreaded spermatocytes. Topo IIα appears enriched at centromeres and also along the chromatid axes (arrows), while SYCP3 is accumulated at the inner centromere domain and also appears at the interchromatid domain, although the labelling interrupts at the chiasma sites (arrowheads). Scale bar, 2.5 μm (JPEG 458 kb)
Fig. S2
Relative distribution of Topo IIα and INCENP during chromosome congression to the metaphase II plate. Double immunolabelling of Topo IIα (green) and INCENP (red) on squashed prometaphase II (a, b) and metaphase II (c, d) spermatocytes. INCENP redistributes from a band traversing the inner centromere domain (a, b) to a single signal at the kinetochore region (c, d), while Topo IIα persists as a TOCOS, as indicated in the schematic illustrations. Scale bar, 0.5 μm (JPEG 300 kb)
Fig. S3
Distribution of PICH and Topo IIα in early anaphase. Double immunolabelling of PICH (green) and Topo IIα (red) and chromatin counterstaining with DAPI (blue) on Pam212 early anaphases. Elongated PICH strands are observed between Topo IIα signals at the centromeres. Scale bar, 3 μm (JPEG 1804 kb)
Fig. S4
Topo IIα distribution during spermatogonial mitosis. a–h Double immunolabelling of Topo IIα (green) and kinetochores (ACA; red) and chromatin counterstaining with DAPI (blue) in spermatogonial prophase and metaphase from squashed seminiferous tubules. i–l Immunolabelling of Topo IIα (green) and chromatin counterstaining with DAPI (blue) on a spreaded spermatogonial metaphase. Topo IIα appears enriched at the centromere regions and faintly represented at the chromatid axes. Two enlarged chromosomes are shown together with a schematic illustration (k, l). Scale bars, 3 μm in h and 5 μm in j (JPEG 1427 kb)
Fig. S5
Apoptosis after Topo IIα inhibition with ET during meiosis I and meiosis II. Double immunolabelling of Topo IIα (green) and kinetochores (ACA; red) and chromatin counterstaining with DAPI (blue) on squashed spermatocytes. Positive immunoreactivity can only be detected in live metaphase I (a) and metaphase II (k) spermatocytes. b–f Apoptotic metaphase I spermatocytes with hypercompacted chromosomes without apparent univalents (b, e) and with univalents (arrowheads in c, d and f). g, h Apoptotic anaphase I/telophase I spermatocytes with lagging chromosomes (g) and without lagging chromosomes (h). i, j Apoptotic metaphase I spermatocytes with fragmented chromosomes. l–o Apoptotic metaphase II spermatocytes with aligned chromosomes (l–n) and misaligned chromosomes (o). p–y Apoptotic anaphase II/telophase II spermatocytes without lagging chromosomes (p, r, u, w, x), with lagging chromosomes (arrowheads) and chromatin bridges (arrows) (q, s, t, v) and with fragmented chromosomes (y). Scale bar, 3 μm (JPEG 1572 kb)
Fig. S6
TUNEL assay after Topo IIα inhibition with ET. TUNEL assay (green) and chromatin counterstaining with DAPI (blue) on squashed spermatocytes after 24 h of ET treatment. Apoptotic cells correspond to those with hypercondensed chromosomes. The image is a projection of different focal planes throughout the cell volume. Scale bar, 5 μm (JPEG 478 kb)
Movie 1
Metaphase I spermatocyte shown in Fig. 1f–h. Double immunolabelling of Topo IIα (green) and kinetochores (red) and chromatin counterstaining with DAPI (blue) (MOV 469 kb)
Movie 2
Metaphase II spermatocyte shown in Fig. 3g–i. Double immunolabelling of Topo IIα (green) and kinetochores (red) and chromatin counterstaining with DAPI (blue) (MOV 259 kb)
Early anaphase II spermatocyte. Immunolabelling of Topo IIα (green) and chromatin counterstaining with DAPI (blue) (MOV 812 kb)
Anaphase I and telophase I spermatocytes after ET treatment shown in Fig. 6e–l. Double immunolabelling of Topo IIα (green) and kinetochores (red) and chromatin counterstaining with DAPI (blue) (MOV 2 mb)
Anaphase II spermatocyte after ET treatment shown in Fig. 7e–h. Double immunolabelling of Topo IIα (green) and kinetochores (red) and chromatin counterstaining with DAPI (blue) (MOV 1.03 mb)
Rights and permissions
About this article
Cite this article
Gómez, R., Viera, A., Berenguer, I. et al. Cohesin removal precedes topoisomerase IIα-dependent decatenation at centromeres in male mammalian meiosis II. Chromosoma 123, 129–146 (2014). https://doi.org/10.1007/s00412-013-0434-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-013-0434-9