Abstract
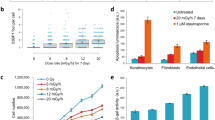
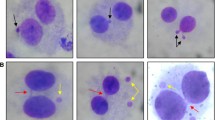
Chromosome damage is related to DNA damage and erroneous repair. It can cause cell dysfunction and ultimately induce carcinogenesis. Histone acetylation is crucial for regulating chromatin structure and DNA damage repair. Ionizing radiation (IR) can alter histone acetylation. However, variations in histone acetylation in response to IR exposure and the relationship between histone acetylation and IR-induced chromosome damage remains unclear. Hence, this study investigated the variation in the total acetylation levels of H3 and H4 in human lymphocytes exposed to 0–2 Gy 60Co γ-rays. Suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor, was added to modify the histone acetylation state of irradiated cells. Then, the total acetylation level, enzyme activity, dicentric plus centric rings (dic + r) frequencies, and micronucleus (MN) frequencies of the treated cells were analyzed. Results indicated that the acetylation levels of H3 and H4 significantly decreased at 1 and 24 h, respectively, after radiation exposure. The acetylation levels of H3 and H4 in irradiated groups treated with SAHA were significantly higher than those in irradiated groups that were not treated with SAHA. SAHA treatment inhibited HDAC activity in cells exposed to 0–1 Gy 60Co γ-rays. SAHA treatment significantly decreased dic + r/cell and MN/cell in cells exposed to 0.5 or 1.0 Gy 60Co γ-rays relative to that in cells that did not receive SAHA treatment. In conclusion, histone acetylation is significantly affected by IR and is involved in chromosome damage induced by 60Co γ-radiation.




Similar content being viewed by others
References
Acharya MR, Sparreboom A, Venitz J, Figg WD (2005) Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol 68(4):917–932
Barjaktarovic Z, Merl-Pham J, Azimzadeh O, Kempf SJ, Raj K, Atkinson MJ, Tapio S (2017) Low-dose radiation differentially regulates protein acetylation and histone deacetylase expression in human coronary artery endothelial cells. Int J Radiat Biol 93(2):156–164
Battu A, Ray A, Wani AA (2011) ASF1A and ATM regulate H3K56-mediated cell-cycle checkpoint recovery in response to UV irradiation. Nucleic Acids Res 39(18):7931–7945
Blattmann C, Oertel S, Thiemann M, Dittmar A, Roth E, Kulozik AE, Ehemann V, Weichert W, Huber PE, Stenzinger A, Debus J (2015) Histone deacetylase inhibition sensitizes osteosarcoma to heavy ion radiotherapy. Radiat Oncol 10:146
Bull CF, Mayrhofer G, Zeegers D, Mun GL, Hande MP, Fenech MF (2012). Folate deficiency is associated with the formation of complex nuclear anomalies in the cytokinesis-block micronucleus cytome assay. Environ Mol Mutagen 53(4):311–323
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10(5):295–304
Cerna D, Camphausen K, Tofilon P (2006) Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol 73:173–204
Chen CC, Tyler J (2008) Chromatin reassembly signals the end of DNA repair. Cell Cycle 7(24):3792–3797
Di Tomaso MV, Gregoire E, Martínez-López W (2017) Effects of valproic acid on radiation-induced chromosomal aberrations in human lymphocytes. Genome Integr 8:4
Drummond D, Noble C, Kirpotin D, Guo Z, Scott G, Benz C (2005) Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45:495–528
Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455(1–2):81–95
Fenech M (2010) The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry. Health Phys 98(2):234 – 43
Gibney ER, Nolan CM (2010) Epigenetics and gene expression. Heredity 105(1):4–13
Gong F, Miller KM (2013) Mammalian DNA repair: HATs and HDACs make their mark through histone acetylation. Mutat Res 750(1–2):23–30
Groth A, Rocha W, Verreault A, Almouzni G (2007) Chromatin challenges during DNA replication and repair. Cell 128(4):721 – 33
Guo R, Chen J, Mitchell DL, Johnson DG (2011) GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res 39(4):1390–1397
Hefferin ML, Tomkinson AE (2005) Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair 4(6):639–648
Hoeijmakers JH (2001) DNA repair mechanisms. Maturitas 38(1):17–22 (discussion 22–23)
Hsiao KY, Mizzen CA (2013) Histone H4 deacetylation facilitates 53BP1 DNA damage signaling and double-strand break repair. J Mol Cell Biol 5(3):157–165
Hunt CR, Ramnarain D, Horikoshi N, Iyengar P, Pandita RK, Shay JW, Pandita TK (2013) Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat Res 179(4):383–392
International Atomic Energy Agency (2011) Cytogenetic dosimetry: application in preparedness for and response to radiation emergencies. IAEA, Vienna
Jazayeri A, McAinsh AD, Jackson SP (2004) Saccharomyces cerevisiae Sin3p facilitates DNA double-strand break repair. PNAS 101:1644–1649
Jeggo PA, Lobrich M (2006) Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability. DNA Repair 5(9–10):1192–1198
Li A, Yu Y, Lee SC, Ishibashi T, Lees-Miller SP, Ausio J (2010) Phosphorylation of histone H2A.X by DNA-dependent protein kinase is not affected by core histone acetylation, but it alters nucleosome stability and histone H1 binding. J Biol Chem 285(23):17778–17788
Mahaney BL, Meek K, Lees-Miller SP (2009) Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J 417(3):639–650
Maroschik B, Gurtler A, Kramer A, Rossler U, Gomolka M, Hornhardt S, Mortl S, Friedl AA (2014) Radiation-induced alterations of histone post-translational modification levels in lymphoblastoid cell lines. Radiat Oncol 9:15
Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP (2010) Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol 17(9):1144–1151
Niewolik D, Pannicke U, Lu H, Ma Y, Wang L, Kulesza P, Zandi E, Lieber M, Schwarz K (2006) DNA-PKcs dependence of Artemis endonucleolytic activity, differences between hairpins and 5′ or 3′ overhangs. J Biol Chem 281:33900–33909
Ogiwara H, Ui A, Otsuka A, Satoh H, Yokomi I, Nakajima S, Yasui A, Yokota J, Kohno T (2011) Histone acetylation by CBP and p300 at double-strand break sites facilitates SWI/SNF chromatin remodeling and the recruitment of non-homologous end joining factors. Oncogene 30(18):2135–2146
Price BD, D’Andrea AD (2013) Chromatin remodeling at DNA double-strand breaks. Cell 152(6):1344–1354
Ramanathan B, Smerdon MJ (1989) Enhanced DNA repair synthesis in hyperacetylated nucleosomes. J Biol Chem 264(19):11026–11034
Roberts SA, Ramsden DA (2007) Loading of the nonhomologous end joining factor, Ku, on protein-occluded DNA ends. J Biol Chem 282(14):10605–10613
Rossetto D, Truman AW, Kron SJ, Cote J (2010) Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res 16(18):4543–4552
Rothkamm K, Kruger I, Thompson LH, Lobrich M (2003) Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 23(16):5706–5715
Seo SK, Jin HO, Woo SH, Kim YS, An S, Lee JH, Hong SI, Lee KH, Choe TB, Park IC (2011) Histone deacetylase inhibitors sensitize human non-small cell lung cancer cells to ionizing radiation through acetyl p53-mediated c-myc down-regulation. J Thorac Oncol 6(8):1313–1319
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ, Cote J, Pandita TK (2010) MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol 30(14):3582–3595
Sun J, Lee KJ, Davis AJ, Chen DJ (2012) Human Ku70/80 protein blocks exonuclease 1-mediated DNA resection in the presence of human Mre11 or Mre11/Rad50 protein complex. J Biol Chem 287(7):4936–4945
Tjeertes JV, Miller KM, Jackson SP (2009) Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J 28(13):1878–1889
van Attikum H, Gasser SM (2009) Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol 19(5):207–217
Verreault A (2000) De novo nucleosome assembly: new pieces in an old puzzle. Genes Dev 14(12):1430–1438
Wyman C, Kanaar R (2004) Homologous recombination: down to the wire. Curr Biol 14(15):R629–R631
Zhang X, Kluz T, Gesumaria L, Matsui MS, Costa M, Sun H (2016) Solar simulated ultraviolet radiation induces global histone hypoacetylation in human keratinocytes. PLoS One 11(2):e0150175
Zhao H, Lu X, Li S, Chen DQ, Liu QJ (2014) Characteristics of nucleoplasmic bridges induced by 60Co gamma-rays in human peripheral blood lymphocytes. Mutagenesis 29(1):49–54
Zhong HM, Ding QH, Chen WP, Luo RB (2013) Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-κB nuclear translocation. Int Immunopharmacol 17(2):329–335
Acknowledgements
All authors wish to thank Drs. De-Qing Chen and Ling Gao for their important suggestions. This study was funded by National Natural Science Foundation of China (No. 81573081 to Q.-J. L.) and Youth Science Research Foundation of NIRP, China CDC (No. 2017-002 to X.-L. T.).
Funding
This study was funded by National Natural Science Foundation of China (No. 81573081 to Q.-J. L.) and Youth Science Research Foundation of NIRP, China CDC (No. 2017-002 to X.-L. T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, XL., Lu, X., Feng, JB. et al. Alterations in histone acetylation following exposure to 60Co γ-rays and their relationship with chromosome damage in human lymphoblastoid cells. Radiat Environ Biophys 57, 215–222 (2018). https://doi.org/10.1007/s00411-018-0742-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-018-0742-9