Abstract
Background
Mannose-binding lectin (MBL) plays an important role in the innate immune response. In addition to activating the complement, MBL can induce cytokine production and contribute to a deleterious inflammatory response with severe A(H1N1)pdm09 virus infection. Our aim was to determine if serum MBL levels correlate with the risk of mortality in intensive care units (ICU) patients with A(H1N1)pdm09 infection.
Methods
Prospective observational study was performed in ICU patients with acute respiratory distress syndrome due to influenza A(H1N1)pdm09 virus. Demographic characteristics and severity indices were recorded at ICU admission. MBL was assayed from blood drawn at influenza diagnosis within 24–48 h following the ICU admission. Outcomes were compared according to MBL levels. Results are expressed as median and interquartile range.
Results
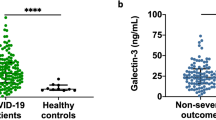
Serum MBL levels were studied in 27 patients (age: 56 [IQR 29] years) with severe A(H1N1)pdm09 infection and in 70 healthy controls. Median admission SAPSII and SOFA scores were 49 [IQR 26] and 12 [IQR 5], respectively. Mortality rate after a 30-day was 37%. MBL was significantly higher in non-survivors (3741 [IQR 2336] ng/ml) vs survivors (215 [IQR 1307] ng/ml), p = 0.006, as well as control group (1814 [IQR 2250] ng/ml), p = 0.01. In contrast, MBL levels in survivors group were significantly lower than the controls group (215 [IQR 1307] ng/ml vs. 1814 [IQR 2250] ng/ml, p = 0.005). MBL cut-off > 1870 ng/ml had a sensitivity of 80% and a specificity of 88.2% for mortality [AUC = 0.82 (95% CI 0.63–0.94)]. Kaplan–Meier analysis demonstrated a strong association between MBL levels and mortality (log-rank 7.8, p = 0.005). MBL > 1870 ng/ml was independently associated with mortality (HR = 8.7, 95% CI 1.2–29.1, p = 0.007).
Conclusions
This study shows that baseline MBL > 1870 ng/ml is associated with higher mortality in ICU patients with severe A(H1N1)pdm09 infection.
Similar content being viewed by others
Background
Severe primary influenza pneumonia can progress to moderate or to severe acute respiratory distress syndrome (ARDS) [1]. Since the 1918 influenza pandemic, “swine fluA” and, more recently, pandemic influenza A(H1N1)pdm09, led to a significant proportion of patients with ARDS [2, 3]. Pandemic influenza A(H1N1)pdm09 is associated with a severe outcome, especially in patients with risk factors such as obesity, metabolic syndrome, pregnancy and underlying comorbidities. Although effective treatments for influenza and its complications exist, identifying early predictive factors of poor outcome in ARDS patients with influenza A(H1N1)pdm09 infection remains relevant [4]. Some studies have shown that A(H1N1)pdm09 can cause a “cytokine storm” with an excessive inflammatory immune response, and this could be a key factor involved in the severe outcome with this virus flu [5,6,7].
Mannose-binding lectin (MBL) is a glycoprotein produced by the liver and belongs to the collectin subfamily of C-type lectins. MBL is a key component of innate immunity [8,9,10], as it activates complement, promotes opsonophagocytosis, and induces the production of pro-inflammatory cytokines. In inflammatory conditions, MBL can leave the blood stream via vascular leakage. In an experimental study, Ling et al. showed that MBL wild-type mice had more severe lung disease with enhanced production of pro-inflammatory cytokines and chemokines than MBL knock-out mice during A(H1N1)pdm09 virus infection, suggesting that MBL may contribute to disease severity. In their study, MBL contributed to a deleterious inflammatory response by upregulating the pro-inflammatory cytokines [11].
Therefore, the aim of the present study was to determine whether levels of serum MBL are associated with mortality in critically ill patients with severe A(H1N1)pdm09 viral pneumonia.
Materials and Methods
Patients
This was a prospective, observational study carried out in the adult intensive care units (ICU) of Amiens University Hospital, France, between September 2009 and March 2016 in all patients in whom influenza A(H1N1)pdm09 virus infection was diagnosed during or 1-week prior to the ICU stay.
Diagnosis of influenza A(H1N1)pdm09 infection was based on the presence of influenza or influenza-like illness, pneumonia, bilateral pulmonary infiltrates, and a positive result on a real-time polymerase chain reaction (RT-PCR) tests for microbiological confirmation of H1N1 influenza.
Demographic and clinical data at admission and during the ICU stay were recorded from the medical files of each patient and collected in a database to evaluate variables potentially associated with in-hospital mortality. Data baselines were recorded at admission. Mechanical ventilation, extracorporeal membrane oxygenation (ECMO) requirements, and the use of vasopressor drugs were noted during ICU stay. To determine illness severity, SAPSII and SOFA scoring systems were applied to all patients within 24 h of ICU admission.
This study was approved by the Institutional Review Board for Human Subjects. Patients or relatives provided their informed consent. The serum MBL of an historical control group was used to compare the levels of survivors and non-survivors.
Virus Detection
Nasopharyngeal or bronchoalveolar lavage fluid (BALF) samples were tested for the presence of A(H1N1)pdm09 virus using a RT-PCR kit according to the manufacturer’s instructions (xTAG RVP Fast assay, Luminex 97 Molecular Diagnostics, Theradiag™).
MBL Levels Determination
When a patient met criteria for ARDS, the closest residual blood sample taken on the same day was obtained from the central hospital laboratory in the 24–48 h following the intubation or in the acute phase of the viral infection.
MBL serum concentrations were assayed by an Oligomer ELISA kit (BioPorto®) according to the manufacturer’s instructions. The lower limit of detection of the assay was 0.02 ng/ml. Patient serum was diluted 1:100 in the diluent buffer and incubated in the wells of ELISA plates for 1 h. After three washes in buffer, the plates were incubated for 1 h at room temperature with a secondary peroxidase-conjugated anti-MBL antibody. After another three further washes, the plates were incubated for 30 min with 200 µl of tetramethylbenzidine (TMB) at room temperature in the dark. The reaction was stopped by the addition of 100 µl of a-ready-to-use stop solution before measuring optical density at 450/620 nm. Serum MBL was quantified using a calibration curve including eight ready-to-use MBL standards with a range of 0–40 ng/ml.
Although there is no universally accepted cut-off concentration, MBL deficiency is defined by a serum MBL concentration ≤ 500 ng/ml. MBL deficiency is present in 20–30% of the population, making MBL deficiency the most common hereditary immunodeficiency, as also describe by Eisen et al. [12,13,14].
Statistical Analysis
Results are expressed as median [interquartile range (IQR)] or number (percent). Survivors were compared with non-survivors using the Mann–Whitney U test for continuous variables and Chi2 test for categorical variables with Yates’ correction or Fisher’s exact test if necessary. A correlation was searched between MBL and inflammatory parameters using spearman test.
A receiver operating characteristic (ROC) curve was constructed to assess the impact of MBL levels on mortality. The Youden index (J) was used to detect the best MBL cut-off for survival analysis. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated for this cut-off. Kaplan–Meier analysis was performed to compare the outcome of groups according to the MBL cut-off. Survival curves were compared using a log-rank test. A multivariate Cox model (backward Wald) was constructed to identify independent factors associated with mortality in this population. Adjusted Hazard Ratios (aHR) and their 95% confidence intervals [95% CI] were calculated. A p value < 0.05 was considered to be statistically significant. Statistical analyses were performed using PASW Statistics 18 (IBM Inc., Chicago, IL, USA) and MedCalc 16.4.3 software (MedCalc Software, Ostend, Belgium) softwares.
Results
Patients Characteristics
Twenty-seven patients were admitted to the ICU with severe pneumonia and with a high probability of viral infection or a previously confirmed diagnosis (median age 56 [IQR 29] years, sex ratio 17 men/10 women). All patients had a confirmed diagnosis of influenza A(H1N1)pdm09 virus infection. They had signs of viral infection, including fever, myalgia, and cough, and were treated with oseltamivir (150 mg/day) for 7 days. All patients with pulmonary symptoms received empirical antimicrobial therapy with ceftriaxone and rovamycin or levofloxacin on admission and this was subsequently adapted to any documented bacterial infection if positive. The median admission SAPS II and SOFA scores were 49 [IQR 26] and 12 [IQR 5], respectively. On admission, 23 patients (85.2%) had severe ARDS and all patients were mechanically ventilated. Thirteen patients (48.1%) needed rescue therapy and received ECMO for refractory hypoxemia. The median length of ICU stay was 22 [IQR 23] days. The 30-day mortality rate was 37% (n = 10/27). Five patients died from severe hypoxemia and organ failure despite oxygenation or ECMO; six patients died from multiorgan failure, due to Aspergillosis co-infections in 4 patients, bacteremia in one patient and mechanical ventilation pneumonia for one other patient.
Comparison demographic and clinical characteristics between survivors and non-survivors is shown in Table 1. As expected, non-survivors were older and had higher severity scores on admission. The control group comprised 70 healthy subjects (HS) among blood donors attending the Regional Center for Blood Transfusion (Lille, France).
Correlation Between Serum MBL Levels and Mortality in Critically Ill A(H1N1)pdm09 Patients
Serum MBL levels were significantly higher in non-survivors (3741 [IQR 2336] ng/ml) than in survivors (215 [IQR 1307] ng/ml]), p = 0.006, as well as in controls group (1814 [IQR 2250] ng/ml), p = 0.01. Oppositely, the serum MBL levels in survivors group were significantly lower than the controls group (215 [IQR 1307] ng/ml vs 1814 [IQR 2250] ng/ml] (p = 0.005), respectively (Fig. 1). The ROC analysis for mortality is shown in Fig. 2 (Area under the curve (AUC) = 0.82 [95% CI 0.63–0.94]). The Youden index (J) was 0.68 for a MBL cut-off > 1870 ng/ml. Sensitivity was 80% [95% CI 44.4–97.5%]; specificity was 88.2% [95% CI 63.6–98.5%]; NPV was 88.2% [95% CI 63.6–98.5%]; PLR was 6.8[95% CI 1.8–25.9]; and NLR was 0.23 [95% CI 0.08–0.80]. Kaplan–Meier survival analyses are shown in Fig. 3. Patients with serum MBL > 1870 ng/ml had a significantly lower 30-day survival estimate than patients with serum MBL ≤ 1870 ng/ml (p = 0.005). SAPSII score > 22, SOFA score > 12, and MBL > 1870 ng/ml were entered into a multivariate Cox model. Only MBL > 1870 ng/ml remained independently associated with mortality in our population (aHR = 8.7 [95% CI 1.2–29.1], p = 0.007). No relationship was evidenced between MBL and inflammatory parameters as WBC count (rho = 0.08, p = 0.93), PMN count (rho = 0.03, p = 0.97), lymphocytes count (rho = 0.05, p = 0.24), platelets count (rho = 0.02, p = 0.93), C reactive protein (rho = 0.01, p = 0.27), and procalcitonin (rho = 0.01, p = 0.61).
Discussion
Pandemic influenza A(H1N1)pdm09 virus causes significant morbidity and mortality in humans. Some studies have shown that influenza A(H1N1)pdm09 infection can induce dysregulation of the immune system, such as cytokine storm and excessive inflammatory infiltrates, leading to lung injury [11]. Among these factors, excessive cytokine production is considered to be the key contributor in severe response during influenza A(H1N1)pdm09 virus infection [5]. MBL contributes to a deleterious inflammatory response by upregulating the pro-inflammatory response [11]. In our study, we determine the serum levels of MBL in critically ill patients with severe A(H1N1)pdm09 infection and analyzed the correlation between plasmatic MBL levels and the risk of mortality. In our study, a serum MBL level > 1870 ng/ml was independently associated with mortality in ICU patients with influenza A(H1N1)pdm09 virus infection and therefore could predict an increased mortality at 30 days.
MBL belongs to the collectin family and functions as a pattern-recognition molecule recognizing a wide range of pathogens. It plays an important role in innate immunity [10]. MBL may represent an important barrier to the establishment of influenza virus infection in epithelial cells. The antiviral activity of MBL against influenza viruses remains controversial. Previous in vitro studies have suggested that MBL exhibits anti-influenza virus function by direct neutralization after binding to mannose-rich glycans on haemagglutinin (HA) and neuraminidase (NA) glycoproteins [15,16,17]. However, this needs to be balanced as the antiviral function of MBL may vary among different strains of influenza viruses, depending on the number of potential glycosylation sites on the viral haemagglutinin globular domain. MBL has been shown to inhibit influenza A seasonal H3N2 and seasonal H1N1 viruses through direct neutralization, indirect opsonization, and complement activation, but limited antiviral effect against H7N9 virus [18]. In contrast, MBL can bind to influenza A(H1N1)pdm09 virus but fails to inhibit its infection in human lung epithelial cell line. Ling et al. have demonstrated in an experimental study that upon A(H1N1)pdm09 infection, MBL is internalized with the virus into the cell, where it may associate with TLR3 to further amplify the NF-κB signaling and augment the cytokine production in the human lung epithelial cells (supplementary data). Their findings advocate the adverse immunomodulating role of MBL during A(H1N1)pdm09 infection by upregulating pro-inflammatory response [11] or may contribute to the pathogenesis of inflammatory-induced vascular damage and organ failure, as observed in patients undergoing solid organ transplantation, leading to a harmful effect of an excessive MBL expression [19].
In our study, serum MBL concentrations observed were very variable from one patient to another, but clearly lower levels in the survivors group than the non-survivors group comparing to the control group (Fig. 1). This heterogeneous distribution in the control group can be explained by individual variations in serum MBL levels that may reflect gene polymorphism ranging from (i) homozygotes with a mean MBL concentrations of 2660 ng/ml (range 40–6790 ng/ml), (ii) heterozygotes with mean MBL concentrations of 830 ng/ml (range 0–4460 ng/ml, and (iii) compound heterozygotes and mutant homozygotes with mean MBL concentrations of 40 ng/ml (range 0–300 ng/ml). Genotype is one of the determinant factors of serum MBL concentrations. However, MBL levels vary significantly among individuals with the same genotype, and the serum MBL concentration may more accurately reflects downstream effector function than genotype. MBL levels do not vary with respect to age, sex, physical exercise, season, time of day, or liver function [16] [20] and are stable during storage at −80 °C through a number of freeze–thaw cycles. Despite these differences in MBL concentrations due to genetic variations, the decrease in serum MBL levels due to MBL consumption during pathogen binding is compensated by an increase in MBL gene transcription during acute infection [12, 13].
Usually, MBL acts as a first line of innate immune response against microbial infections, and it may regulate pro-inflammatory cytokine and chemokine release from phagocytes in response to bacterial and fungal stimulations [21,22,23]. Many studies have shown that low serum MBL levels are associated with a susceptibility to bacterial, fungal and viral, and leads to a worse outcome [14, 24]. On the other side, sporadic reports have not found a clear association between MBL deficiency and increased rate of infectious episodes [25,26,27]. Despite these informations, little is known about the MBL immunomodulatory role in established influenza infection. Chang et al. demonstrate in a mouse study that MBL plays a key role in clearing influenza A virus and lung homeostasis. In the other side, this study suggests that MBL deficiency maybe a risk factor in influenza A virus infection. Our study included adult patients with severe ARDS with very high mortality score (SAPSII and SOFA scores) that can explain high serum MBL levels as a response to an acute phase response as demonstrated by Patersen et al. [28].
Other glycoproteins are involved in the regulation of innate immunity and the antiviral responses such as SP-D. The high serum level of SP-D was described as a predictor of poorer outcomes in ICU patients with ARDS associated with influenza A(H1N1)pdm09 virus infection [29]. In our study, the serum MBL levels > 1870 ng/ml were independently associated with mortality in critically ill patients with severe influenza A(H1N1)pdm09 virus infection. Moreover, the serum MBL levels are not affected by any respiratory chronic disease in contrast to SP-D which could be a prognostic biomarker of clinical exacerbations and COPD progression as presented by Sin et al. [30].
Our study has several limitations. The sample size was restricted by the study’s focus on critically ill patients with virologically confirmed influenza A(H1N1)pdm09 virus. Therefore, we should be cautions with our interpretation of data. Another limitation is the absence of a control group of patients with ARDS associated with respiratory viruses. It would be of interest to determine whether MBL variations are related to others respiratory virus or not. As for all biomarkers, the range of MBL concentrations is very wide and it is difficult to know if a patient is in the group with a better prognosis or not. Larger studies should better define this range.
Conclusions
Serum MBL levels > 1870 ng/ml were independently associated with mortality in critically ill patients with severe influenza A(H1N1)pdm09 virus infection. This protein could be a potential early biomarker of prognosis. Future studies are required to confirm these results.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
Area under the curve
- BALF:
-
Bronchoalveolar lavage fluid
- COPD:
-
Chronic obstructive pulmonary disease
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- MBL:
-
Mannose-binding lectin
- NLR:
-
Negative likelihood ratio
- NPV:
-
Negative predictive value
- OD:
-
Optical density
- PLR:
-
Positive likelihood ratio
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- RT-PCR:
-
Real-time polymerase chain reaction
- SAPS II:
-
Simplified acute physiology score
- SOFA:
-
Sequential organ failure assessment
References
Ferguson ND, Fan E, Camporota L et al (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38:1573–1582. https://doi.org/10.1007/s00134-012-2682-1
Olson DR, Simonsen L, Edelson PJ, Morse SS (2005) Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci USA 102:11059–11063. https://doi.org/10.1073/pnas.0408290102
Andreasen V, Viboud C, Simonsen L (2008) Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis 197:270–278. https://doi.org/10.1086/524065
Mulrennan S, Tempone SS, Ling ITW et al (2010) Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS ONE 5:e12849. https://doi.org/10.1371/journal.pone.0012849
Lee N, Wong CK, Chan PKS et al (2011) Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS ONE 6:e26050. https://doi.org/10.1371/journal.pone.0026050
Shi X, Zhou W, Huang H et al (2013) Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit Care 17:R301. https://doi.org/10.1186/cc13171
Rondina MT, Tatsumi K, Bastarache JA, Mackman N (2016) Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza A/H1N1 infection. Crit Care Med 44:e574–e578. https://doi.org/10.1097/CCM.0000000000001584
Kawasaki T (1999) Structure and biology of mannan-binding protein, MBP, an important component of innate immunity. Biochim Biophys Acta 1473:186–195
Fujita T, Matsushita M, Endo Y (2004) The lectin-complement pathway—its role in innate immunity and evolution. Immunol Rev 198:185–202
Ip WKE, Takahashi K, Ezekowitz RA, Stuart LM (2009) Mannose-binding lectin and innate immunity. Immunol Rev 230:9–21. https://doi.org/10.1111/j.1600-065X.2009.00789.x
Ling MT, Tu W, Han Y et al (2012) Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. J Infect Dis 205:44–53. https://doi.org/10.1093/infdis/jir691
Perez-Castellano M, Peñaranda M, Payeras A et al (2006) Mannose-binding lectin does not act as an acute-phase reactant in adults with community-acquired pneumococcal pneumonia. Clin Exp Immunol 145:228–234. https://doi.org/10.1111/j.1365-2249.2006.03140.x
Dean MM, Minchinton RM, Heatley S, Eisen DP (2005) Mannose binding lectin acute phase activity in patients with severe infection. J Clin Immunol 25:346–352. https://doi.org/10.1007/s10875-005-4702-1
Eisen DP, Dean MM, Boermeester MA et al (2008) Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis 47:510–516. https://doi.org/10.1086/590006
Hartshorn KL, Sastry K, White MR et al (1993) Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest 91:1414–1420. https://doi.org/10.1172/JCI116345
Kase T, Suzuki Y, Kawai T et al (1999) Human mannan-binding lectin inhibits the infection of influenza A virus without complement. Immunology 97:385–392
Anders EM, Hartley CA, Reading PC, Ezekowitz RA (1994) Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol 75(Pt 3):615–622. https://doi.org/10.1099/0022-1317-75-3-615
Guo J, Cao Y, Qin K et al (2015) Limited effect of recombinant human mannose-binding lectin on the infection of novel influenza A (H7N9) virus in vitro. Biochem Biophys Res Commun 458:77–81. https://doi.org/10.1016/j.bbrc.2015.01.070
Berger SP, Roos A, Mallat MJK et al (2007) Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J Am Soc Nephrol 18:2416–2422. https://doi.org/10.1681/ASN.2007030262
Homann C, Garred P, Hasselqvist P et al (1995) Mannan-binding protein and complement dependent opsonization in alcoholic cirrhosis. Liver 15:39–44
Jack DL, Jarvis GA, Booth CL et al (2001) Mannose-binding lectin accelerates complement activation and increases serum killing of Neisseria meningitidis serogroup C. J Infect Dis 184:836–845. https://doi.org/10.1086/323204
Nadesalingam J, Dodds AW, Reid KBM, Palaniyar N (2005) Mannose-binding lectin recognizes peptidoglycan via the N-acetyl glucosamine moiety, and inhibits ligand-induced proinflammatory effect and promotes chemokine production by macrophages. J Immunol 175:1785–1794
Wang M, Wang F, Yang J et al (2013) Mannan-binding lectin inhibits Candida albicans-induced cellular responses in PMA-activated THP-1 cells through Toll-like receptor 2 and Toll-like receptor 4. PLoS ONE 8:e83517. https://doi.org/10.1371/journal.pone.0083517
Garcia-Laorden MI, Sole-Violan J, Rodriguez de Castro F, et al (2008) Mannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adults. J Allergy Clin Immunol 122:368–374, 374–382. https://doi.org/10.1016/j.jaci.2008.05.037
García-Laorden MI, Rodríguez de Castro F, Solé-Violán J et al (2013) The role of mannose-binding lectin in pneumococcal infection. Eur Respir J 41:131–139. https://doi.org/10.1183/09031936.00174111
Wong M, Öhrmalm L, Broliden K et al (2012) Mannose-binding lectin 2 polymorphisms do not influence frequency or type of infection in adults with chemotherapy induced neutropaenia. PLoS ONE 7:e30819. https://doi.org/10.1371/journal.pone.0030819
Dahl M, Tybjaerg-Hansen A, Schnohr P, Nordestgaard BG (2004) A population-based study of morbidity and mortality in mannose-binding lectin deficiency. J Exp Med 199:1391–1399. https://doi.org/10.1084/jem.20040111
Petersen SV, Thiel S, Jensenius JC (2001) The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol 38:133–149
Delgado C, Krötzsch E, Jiménez-Alvarez LA et al (2015) Serum surfactant protein D (SP-D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung 193:25–30. https://doi.org/10.1007/s00408-014-9669-3
Sin DD, Leung R, Gan WQ, Man SP (2007) Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med 7:13. https://doi.org/10.1186/1471-2466-7-13
Acknowledgements
The authors would like to thank all of the patients for their participation in this study. The cooperation of the medical and paramedical staff of intensive care units of the University Hospital, Amiens, France, is gratefully acknowledged.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EZ, RN, TC, and HD contributed to the conception and design of the study. EZ, RN, and TC performed the acquisition of the data. HD performed the statistical analysis. EZ, RN, TC, JM, VJ, JM, CS, SB, and HD contributed to the analysis and interpretation of data, wrote and approved the final manuscript. EZ, RN, TC, JM, VJ, JM, CS, SB, and HD gave agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board for Human Subjects.
Informed consent
Patients or relatives provided their informed consent.
Rights and permissions
About this article
Cite this article
Zogheib, E., Nyga, R., Cornu, M. et al. Prospective Observational Study on the Association Between Serum Mannose-Binding Lectin Levels and Severe Outcome in Critically Ill Patients with Pandemic Influenza Type A (H1N1) Infection. Lung 196, 65–72 (2018). https://doi.org/10.1007/s00408-017-0067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-017-0067-5