Abstract
Objective
Our study aims to determine the influence of smoking or tobacco chewing and the association of Interleukin 6 (IL-6) polymorphism, where G is substituted by A at the position − 596 (IL-6 − 596 G/A) and substitution of G by cytosine (C) at position − 572 (IL-6 − 572 G/C) on the susceptibility of precancerous oral lesions and oral cancer.
Materials and methods
The participants consisted of 250 subjects among which 75 were suffering from oral cancer, 75 subjects with precancerous oral lesions and 100 were healthy controls. Single-nucleotide polymorphism study (SNP) was done by polymerase chain reaction and restriction fragment length polymorphism (PCR–RFLP).
Results
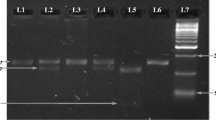
IL-6 − 596 G/A SNP revealed genotypes, GG, and GA in subjects with precancerous oral lesions and oral cancer, and AA genotype was not found in any subject. IL-6 − 596 G/A was strongly associated with oral precancerous lesions but not with oral cancer. The present study reports that smokers carrying GA for IL-6 − 596 G/A were at several folds higher risk of developing oral precancerous lesions. Smokers with GC and CC for IL-6 − 572 G/C were at higher risk of developing oral precancerous lesions. No significant interaction was observed between these habits and IL-6 − 596 G/A and IL-6 − 572 G/C SNP with oral cancer.
Conclusion
The interaction of variant A allele of IL-6 − 596 G/A and C allele of IL-6 − 572 G/C polymorphism with smoking and increases the risk of oral precancerous lesions. Tobacco chewing was not related with IL-6 − 596 G/A or IL-6 − 572 G/C in oral precancerous lesions or oral cancer.
Clinical relevance
The study will help to determine the susceptibility of individuals with smoking or chewing habits to the development of oral precancerous lesion and oral cancer by monitoring the IL-6 SNPs which can be used as a biomarker for risk determination.


Similar content being viewed by others
Data availability
Research data are not shared. The data are not publicly available due to privacy or ethical restrictions.
References
Bray F, Ren JS, Masuyer E, Ferlay J (2013) Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 132:1133–1145
Williams HK (2000) Molecular pathogenesis of oral squamous carcinoma. Mol Pathol 53:165–172
Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R et al (2012) Cancer mortality in India: a nationally representative survey. Lancet 379:1807–1816
Mandal S, Abebe F, Chaudhary J (2014) 174 G/C polymorphism in the interleukin-6 promoter is differently associated with prostate cancer incidence depending on race. Genet Mol Res 13:139–151
Lisa Cheng YS, Jordan L, Gorugantula LM, Schneiderman E, Chen HS, Rees T (2014) Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J Periodontol 85:956–965. https://doi.org/10.1902/jop.2013.130320
Olomolaiye O, Wood NA, Bidwell JL (1998) A novel NlaIII polymorphism in the human IL-6 promoter. Eur J Immunogenet 25:267
Fishman D, Faulds G, Jeffery R, Ali VM, Yudkin JS, Humphries S, Woo P (1998) The effect of novel polymorphisms in interleukin 6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset Juvenile chronic arthritis. J Clin Invest. 102:1369–1376
Nibali L, Fedele S, D’Aiuto F, Donos N (2012) Interleukin-6 in oral diseases: a review. Oral Dis 18:236–243. https://doi.org/10.1111/j.1601-0825.2011.01867.x
Gautam KA, Muktanand T, Sankhwar SN, Goel A, Sankhwar PL, Rajender S (2016) Functional polymorphism in the IL-6 gene promoter and urinary bladder cancer in India. Cytokine 77:152–156
Slattery ML, Curtin K, Baumgartner R, Sweeney C, Byers T, Giuliano AR, Baumgartner KB, Wolff RR (2007) IL-6, aspirin, nonsteroidal anti-inflammatory drugs, and breast cancer risk in women living in the southwestern United States. Cancer Epidemiol Biomark Prev 16:747–755
Jerrard-Dunne P, Sitzer M, Risley P, Buehler A, von Kegler S, Markus HS (2004) Inflammatory gene load is associated with enhanced inflammation and early carotid atherosclerosis in smokers. Stroke 35:2438–2443
Seow A, Ng DP, Choo S, Eng P, Poh WT, Ming T, Wang YT (2006) Joint effect of asthma/atopy and an IL-6 gene polymorphism on lung cancer risk among lifetime non-smoking Chinese women. Carcinogenesis 27:1240–1244
Peng X, Shi J, Sun W, Ruan X, Guo Y, Zhao L, Wang J, Li B (2018) Genetic polymorphisms of IL-6 promoter in cancer susceptibility and prognosis: a meta-analysis. Oncotarget 9:12351–12364. https://doi.org/10.18632/oncotarget.24033
Singh PK, Chandra G, Bogra J, Gupta R, Kumar V, Jain A, Hussain SR, Mahdi AA, Ahmad MK (2015) Association of interleukin-6 genetic polymorphisms with risk of OSCC in Indian population. Met Gen 4:142–151. https://doi.org/10.1016/j.mgene.2015.03.002
Shahi Y, Samadi FM, Mukherjee S (2020) Plasma lipid peroxidation and antioxidant status in patients with oral precancerous lesions and oral cancer. Oral Sci Int 17:2. https://doi.org/10.1002/osi2.1050
Shahi Y, Samadi FM, Mukherjee S (2019) The influence of tumor necrosis factor-alpha gene polymorphism on oxidative stress in patients with oral precancerous lesions and oral cancer. Gene Rep 17:100525. https://doi.org/10.1016/j.genrep.2019.100525
Tsai YY, Lin JM, Wan L, Ju Lin H, Tsai Y, Chun Lee C, Tsai CH, Tsai FJ, Tseng SH (2008) Interleukin gene polymorphisms in age-related macular degeneration. Invest Ophthalmol Vis Sci 49:693–698
Savage SA, Abnet CC, Haque K, Mark SD, Qiao YL, Dong ZW, Dawsey SM, Taylor PR, Chanock SJ (2004) Polymorphisms in interleukin -2,-6, and -10 are not associated with gastric cardia or esophageal cancer in a high-risk Chinese population. Cancer Epidemiol Biomark Prev 13:1547–1549
Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, Munroe DJ, Farrar WL (2005) Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res 65:4673–4682
Hamad AWR, Gaphor SM, Shawagfeh MT, Al-Talabani NG (2011) Study of serum and salivary levels of proinflammatory cytokines, potential biomarkers in the diagnosis of oral squamous cell carcinoma. Acad J Cancer Res 4:47–55
Liao G, Wang Y, Zhou YQ, Li TW, Zeng DQ, Zeng X (2014) Host genetic susceptibility to oral cancer: evidence from meta-analyses and pooled analyses. Oral Dis 20(7):644–649
Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, Suveges D, Vrousgou O, Whetzel PL, Amode R, Guillen JA, Riat HS, Trevanion SJ, Hall P, Junkins H, Flicek P, Burdett T, Hindorff LA, Cunningham F, Parkinson H (2019) The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47(D1):D1005–D1012. https://doi.org/10.1093/nar/gky1120
Sreekumar VN (2019) Global scenario of research in oral cancer. J Maxillofac Oral Surg 18(3):354–359. https://doi.org/10.1007/s12663-018-1166-4
Gomes CC, Gomez RS (2008) MicroRNA and oral cancer: future perspectives. Oral Oncol 44(910–914):44
Wu BH, Xiong XP, Jia J, Zhang WF (2011) MicroRNAs: new actors in the oral cancer scene. Oral Oncol 47:314–319
Hiyama T, Yoshihara M, Tanaka S, Chayama K (2008) Genetic polymorphisms and head and neck cancer risk. Int J Oncol 32(5):945–973
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14(5):365–376
Erdei E, Luo L, Sheng H, Maestas E, White KA, Mackey A, Dong Y, Berwick M, Morse DE (2013) Cytokines and tumor metastasis gene variants in oral cancer and precancer in Puerto Rico. PLoS ONE 8(11):e79187. https://doi.org/10.1371/journal.pone.0079187
Javalera D, Quintero-Ramos A, Medina-Mora Y, Toro-Arreola AD, Franco-Topete RA, Oceguera-Villanueva A, Barragán-Ruiz A, Flores-Márquez MAR, Topete A, Daneri-Navarro A (2020) The -174 G > C and -596 G > A Polymorphisms are not associated with circulating IL-6 levels in breast cancer patients from Jalisco, Mexico. Genet Test Mol Biomark 24:224–228. https://doi.org/10.1089/gtmb.2019.014
Liang J, Liu X, Bi Z, Yin B, Xiao J, Liu H, Li Y (2013) Relationship between gene polymorphisms of two cytokine genes (TNF-a and IL- 6) and occurring of lung cancers in the ethnic group Han of China. Mol Biol Rep 40:1541–1546
Yin YW, Sun QQ, Hu AM, Wang Q, Uu HL, Hou ZZ, Zeng YH, Xu RJ, Shi LB, Ma JB (2012) Associations between interleukin-6 gene -174 C/G and -572 C/G polymorphisms and the risk of gastric cancer: a meta-analysis. J Surg Oncol 106:987–993
Barros SP, Offenbacher S (2009) Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res 88:400–408. https://doi.org/10.1177/0022034509335868
Ram H, Sarkar J, Kumar H, Konwar R, Bhatt ML, Mohammad S (2011) Oral cancer: risk factors and molecular pathogenesis. J Maxillofac Oral Surg 10(2):132–137. https://doi.org/10.1007/s12663-011-0195-z
Rivera C (2015) Essentials of oral cancer. Int J Clin Exp Pathol 8:11884–11894
Kumar M, Nanavati R, Modi TG, Dobariya C (2016) Oral cancer: etiology and risk factors: a review. J Cancer Res Ther 12:458–463. https://doi.org/10.4103/0973-1482.186696
Church TR, Haznadar M, Geisser MS, Anderson KE, Caporaso NE, Le C, Adullah SB, Hecht SS, Oken MM, Ness BV (2010) Interaction of CYP1B1, cigarette-smoke carcinogen metabolism, and lung cancer risk. Int J Mol Epidemiol Genet 1(4):295
Oladipupo OA, Dutta D, Chong NS (2019) Analysis of chemical constituents in mainstream bidi smoke. BMC Chem 13(1):93
Hecht SS (2002) Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol 3(8):461–469
Rodu B, Jansson C (2004) Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med 15(5):252–263
WHO report on the global tobacco epidemic (2008) The MPOWER package. World Health Organization, Geneva
Vairaktaris E, Yiannopoulos A, Vylliotis A, Yapijakis C, Derka S, Vassiliou S, Nkenke E, Serefoglou Z, Ragos V, Tsigris C, Vorris E, Critselis E, Avgoustidis D, Neukam FW, Patsouris E (2006) Strong association of interleukin-6-174 G > C promoter polymorphism with increased risk of oral cancer. Int J Biol Markers 21:246–250
Zhang YM, Mao YM, Sun YX (2015) Genetic polymorphisms of IL-6 and IL-10 genes correlate with lung cancer in never-smoking Han population in China. Int J Clin Exp Med 8:1051–1058
Ge S, Ye P, Li GY, Fu YF, Zhou Q, Huan F, Wang X, Wang WM (2019) Effects of active and passive smoking on salivary cytokines levels in rats: a pilot study. Toxicol Ind Health 35:109–118. https://doi.org/10.1177/0748233718817192
Brailo V, Vucićević-Boras V, Cekić-Arambasin A, Alajbeg IZ, Milenović A, Lukac J (2006) The significance of salivary interleukin 6 and tumor necrosis factor-alpha in patients with oral leukoplakia. Oral Oncol 42:370–373
Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, Totoki Y, Fujimoto A, Nakagawa H, Shibata T, Campbell PJ, Vineis P, Phillips DH, Stratton MR (2016) Mutational signatures associated with tobacco smoking in human cancer. Science 354:618–622. https://doi.org/10.1126/science.aag0299
Acknowledgements
The authors gratefully acknowledge the infrastructure support received from Amity University Uttar Pradesh Lucknow Campus carrying out this study.
Funding
The work was not funded by any funding agency. The infrastructure support was provided by Amity Institute of Biotechnology, Amity University Uttar Pradesh Lucknow Campus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mr. Yadvendra Shahi declares that he has no conflict of interest. Dr Sayali Mukherjee declares that she has no conflict of interest. Dr. Fahad M. Samadi declares that he has no conflict of interest.
Ethics approval
This article contains studies with human participants performed by the authors. All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee of King George Medical University, Lucknow, Uttar Pradesh, India and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All the authors accepted the paper in its present condition and have given their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahi, Y., Mukherjee, S. & Samadi, F.M. Interaction of tobacco chewing and smoking habit with interleukin 6 promoter polymorphism in oral precancerous lesions and oral cancer. Eur Arch Otorhinolaryngol 278, 4011–4019 (2021). https://doi.org/10.1007/s00405-021-06620-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06620-z