Abstract
Purpose
To measure the duration of nasolacrimal transition in patients with Multiple Sclerosis (MS), to compare the findings with the healthy population and to investigate the relationship between MS-related disability and nasolacrimal transition time.
Methods
A total of 73 individuals including 37 patients with relapsing–remitting MS and 36 healthy volunteers were included in the study. In both groups the nasolacrimal transition time was measured using flurosein drops. The degree of disability of MS patients was calculated according to the Expanded Disability Status Scale (EDSS).
Results
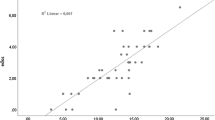
There was a significant difference between MS patients and control group in terms of the median value of nasolacrimal transition time in (240 s (min: 85, max: 724) and 58.5 s (min: 21, max: 428), respectively) (p < 0.001). A positive correlation was found between EDSS scores and nasolacrimal transition time of MS patients (r = 0.384, p = 0.019).
Conclusion
This study showed that nasolacrimal transition time is prolonged in MS patients and there is a relationship between the degree of MS-related disability and the nasolacrimal transition time. This is the first study in the literature on this subject and the results of our study have the potential to shed light on the causes of common infections in MS patients. Long-term prospective studies are needed to further investigate the relationship between prolonged nasolacrimal transition and infections in MS patients.

Similar content being viewed by others
References
Kular L, Jagodic M (2020) Epigenetic insights into multiple sclerosis disease progression. J Intern Med 288:82–102
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391(10130):1622–1636
Persson R, Lee S, Yood MU, Wagner CM, Minton N, Niemcryk S, Lindholm A, Evans AM, Jick SS (2020) Infections in patients diagnosed with multiple sclerosis: a multi-database study. MultSclerRelatDisord 41:101982
Steelman AJ (2015) Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol 19(6):520
Marrodan M, Alessandro L, Farez MF, Correale J (2019) The role of infections in multiple sclerosis. MultScler 25(7):891–901
Wijnands JM, Kingwell E, Zhu F, Zhao Y, Fisk JD, Evans C, Marrie RA, Tremlett H (2017) Infection related health care utilization among people with and without multiple sclerosis. MultScler 23(11):1506–1516
Heath G, Airody A, Gale RP (2017) The ocular manifestations of drugs used to treat multiple sclerosis. Drugs 77:303–311
Zecca C, Nessi F, Bernasconi E, Gobbi C (2009) Ocular toxoplasmosis during natalizumab treatment. Neurology 73(17):1418–1419
Perry HD (2008) Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care 14(3 Suppl):S79-87
Ali MJ, Paulsen F (2020) Human lacrimal drainage system reconstruction, recanalization, and regeneration. Curr Eye Res 45(3):241–252
Prokosch V, Prokosch JE, Julia P, Idelevich EA, Böhm MRR, Thanos S, Stupp T (2014) Bacterial spectrum and antimicrobial susceptibility patterns in acquired and connatal lacrimal duct stenosis. Curr Eye Res 39(11):1069–1075
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonaldcriteria. Lancet Neurol 17(2):162–173
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1446
Jones LT (1961) An anatomical approach to problems of the eyelids and lacrimal apparatus. Arch Ophthalmol 66(1):111–124
Jones LT, Wobig JL (1991) Surgery of the eyelids and lacrimal system. Birmingham, Alabama: Aesculapius, 1976. Munden PM, Kardon RH, Denison CE, Carter KD. Palpebral fissure responses to topical adrenergic drugs. Am J Ophtalmol 111:706–710
Becker BB (1990) Flexible endoscopy in primary dye testing of the lacrimal system. Ophthalmic Surg Lasers Imaging Retina 21(8):577–580
Onerci M (2002) Dacryocystorhinostomy. Diagnosis and treatment of nasolacrimal canal obstructions. Rhinology 40(2):49–65
Örnek N, Dağ E, Örnek K (2015) Corneal sensitivity and tear function in neurodegenerative diseases. Curr Eye Res 40(4):423–428
Miro J, Peña-Sagredo JL, Berciano J, Insua S, Leno C, Velarde R (1990) Prevalence of primary Sjögren’s syndrome in patients with multiple sclerosis. Ann Neurol 27(5):582–584
Annunziata P, De Santi L, Di Rezze S, Millefiorini E, Capello E, Mancardi G, RizM De, Scarpini E, Vecchio R, Patti F (2011) Clinical features of Sjogren’s syndrome in patients with multiple sclerosis. Acta NeurolScand 124(2):109–114
Jones LT (1973) Anatomy of the tear system. Int OphthalmolClin 13(1):3–22
Palakuru JR, Wang J, Aquavella JV (2007) Effect of blinking on tear dynamics. Invest Ophthalmol Vis Sci 48(7):3032–3037
Zadro I, Barun B, Habek M, Brinar VV (2008) Isolated cranial nerve palsies in multiple sclerosis. ClinNeurolNeurosurg 110(9):886–888
Degirmenci E, Erdogan C, Bir LS (2013) Correlation between blink reflex abnormalities and magnetic resonance imaging findings in patients with multiple sclerosis. Acta Neurol Belg 113(3):265–269
Klissurski M, Novachkova S, Pl T, Alexiev F (2009) Orbicularis oculi reflex abnormalities in patients with multiple sclerosis: a clinical, EMG, and MRI investigation. ElectromyogrClinNeurophysiol 49(1):59–63
Brooks JBB, Jardim MR, Papais-Alvarenga RM, Fragoso YD (2015) There is still a role for the blink reflex in the diagnosis and follow-up of multiple sclerosis. ClinNeurophysiol 126(4):743–747
DeAngelis D, Hurwitz J, Mazzulli T (2001) The role of bacteriologic infection in the etiology of nasolacrimal duct obstruction. Can J Ophthalmol 36(3):134–139
Hartikainen J, Lehtonen O-P, Saari KM (1997) Bacteriology of lacrimal duct obstruction in adults. Br J Ophthalmol 81(1):37–40
Li G, Guo J, Liu R, Hu W, Xu L, Wang J, Cai S, Zhang H, Zhu Y (2016) Lacrimal duct occlusion is associated with infectious keratitis. Int J Med Sci 13(10):800–805
Sood AB, Kumar G, Robinson J (2016) Bilateral acute retinal necrosis in a patient with multiple sclerosis on natalizumab. J Ophthalmic Inflamm Infect 6(1):1–4
Naiboglu B, Devecı I, Kalaycık C, Daylan A, Habesoglu TE, Toros SZ, Devecı SE, Egelı E (2010) Effect of nasolacrimal duct obstruction on nasal mucociliary transport. J LaryngolOtol 124(2):166–170
Sahin E, Hamamcı M, KantekinY, (2020) Measurement of mucociliary clearance in the patients with multiple sclerosis. Eur Arch Otorhinolaryngol 277(2):469–473
Gudis DA, Cohen NA (2010) Cilia dysfunction. OtolaryngolClin North Am 43(3):461–472
Vinogradova Y, Hippisley-Cox J, Coupland C (2009) Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract 59(567):e329–e338
Correale J, Fiol M, Gilmore W (2006) The risk of relapses in multiple sclerosis during systemic infections. Neurology 67(4):652–659
Kale N, Magana S, Agaoglu J, Tanik O (2009) Assessment of autonomic nervous system dysfunction in multiple sclerosis and association with clinical disability. Neurol Int 1(5):15–18
Gunal DI, Afsar N, Tanridag T, Aktan S (2002) Autonomic dysfunction in multiple sclerosis: correlation with disease-related parameters. EurNeurol 48(1):1–5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of İnterest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Yozgat Bozok University Clinical Researches Ethic Committee Ref No: 2017-KAEK-189ˍ2019.12.25ˍ14) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dağıstan, H., Hamamcı, M. Nasolacrimal transition time in patients with multiple sclerosis. Eur Arch Otorhinolaryngol 278, 3357–3362 (2021). https://doi.org/10.1007/s00405-021-06603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06603-0