Abstract
Purpose
The purpose of this review is to highlight the benefits of gender-neutral and the nonavalent human papillomavirus vaccination. Human papillomavirus infection is the most commonly sexually transmitted disease and is known to cause several types of cancers, including cervical, vulvar, vaginal, penile, oropharyngeal, anal, and rectal. 5% of cancers every year are attributable to human papillomavirus infection, with cervical cancer the most common and oropharyngeal cancer estimated to surpass the incidence of cervical cancer by 2020.
Methods
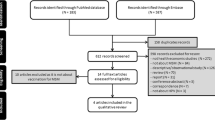
PubMed and MEDLINE were searched using the following search terms: [(human papillomavirus OR HPV) AND (vaccine OR vaccination)] AND [(gardasil OR gardasil9 OR cervarix OR quadrivalent OR nonavalent OR ninevalent) OR (gender neutral OR male)].
Results
There are currently three different types of human papillomavirus vaccinations and range in cover from four to nine different strains known to cause human disease. Most countries currently only supply vaccination to females; however, recent data point towards both a personal benefit as well as a cost-effective population-based benefit with gender-neutral vaccination. Data from female vaccination only have shown the vaccine to be effective in preventing premalignant cervical lesions, and are believed to have the same effect for other human papillomavirus cancers. Male vaccination not only provides personal benefit but also has a “herd effect” for females by preventing the propagation of the virus.
Conclusion
Gender-neutral vaccination provides significant cost-effective benefits for preventing human papillomavirus-related diseases, and this effect is further enhanced by the use of the nonavalent vaccine.
Similar content being viewed by others
References
Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G (2013) A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445(1–2):224–231. https://doi.org/10.1016/j.virol.2013.07.015
Viens LJ (2016) Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morbidity and mortality weekly report 65
Bernard H-U, Burk RD, Chen Z, van Doorslaer K, Hausen Hz, de Villiers E-M (2010) Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401(1):70–79. https://doi.org/10.1016/j.virol.2010.02.002
Schiffman M, Clifford G, Buonaguro FM (2009) Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agents Cancer 4(1):8. https://doi.org/10.1186/1750-9378-4-8
Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, Copeland G, Cozen W, Peters ES, Huang Y, Saber MS, Altekruse S, Goodman MT (2015) US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 107(6):djv086. https://doi.org/10.1093/jnci/djv086
Narisawa-Saito M, Kiyono T (2007) Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci 98(10):1505–1511. https://doi.org/10.1111/j.1349-7006.2007.00546.x
Cancer Trends No 33. HPV-associated cancers (2017). vol 2017. National Cancer Registry Ireland. http://www.ncri.ie/news/article/hpv-associated-cancers-ireland-report-national-cancer-registry
European Public Assessment Report for Gardasil -EMEA/H/C/000703 (2017) European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000703/human_med_000805.jsp. Accessed 31 July 2017
European Public Assessment Report for Gardasil 9-EMEA/H/C/003852 (2017) European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003852/human_med_001863.jsp&mid=WC0b01ac058001d124. Accessed 31 July 2017
European Public Assessment Report for Cervarix-EMEA/H/C/000721 (2017) European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000721/human_med_000694.jsp&mid=WC0b01ac058001d124. Accessed 31 July 2017
Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JML, Cummings T, Donovan B, Fairley CK, Flagg EW, Johnson AM, Kahn JA, Kavanagh K, Kjaer SK, Kliewer EV, Lemieux-Mellouki P, Markowitz L, Mboup A, Mesher D, Niccolai L, Oliphant J, Pollock KG, Soldan K, Sonnenberg P, Tabrizi SN, Tanton C, Brisson M (2015) Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 15(5):565–580. https://doi.org/10.1016/s1473-3099(14)71073-4
Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Group HPS (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374(9686):301–314. https://doi.org/10.1016/S0140-6736(09)61248-4
Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M (2012) Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12(10):781–789. https://doi.org/10.1016/S1473-3099(12)70187-1
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens–Part B: biological agents. Lancet Oncol 10(4):321–322
Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S (2010) Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 202(12):1789–1799. https://doi.org/10.1086/657321
Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, Giuliano AR (2010) Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis 37(6):386–391. https://doi.org/10.1097/OLQ.0b013e3181c94a3b
Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK (2012) Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 307(7):693–703. https://doi.org/10.1001/jama.2012.101
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S (2012) Global burden of human papillomavirus and related diseases. Vaccine 30 Suppl 5:F12–F23. https://doi.org/10.1016/j.vaccine.2012.07.055
de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menendez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andujar M, Castellsague X, Sanchez GI, Nowakowski AM, Bornstein J, Munoz N, Bosch FX, Retrospective International S, Group HPVTTS. (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11):1048–1056. https://doi.org/10.1016/S1470-2045(10)70230-8
Human Papillomavirus (HPV) Vaccine Information (2017) Health service executive. http://www.hse.ie/eng/health/immunisation/pubinfo/schoolprog/hpv/. Accessed 12 Aug 2017
HPV Vaccination Patient Information Leaflet for Men who have Sex with Men (MSM) (2017) Health service executive. http://www.crisispregnancy.ie/wp-content/uploads/2016/09/HPV-Vaccine-Patient-Information-Leaflet-MSM-7Nov2016.pdf. Accessed 12 Aug 2017
Lu B, Kumar A, Castellsague X, Giuliano AR (2011) Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis 11:13. https://doi.org/10.1186/1471-2334-11-13
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D (2011) Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 364(5):401–411. https://doi.org/10.1056/NEJMoa0909537
Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, Lowy DR, Porras C, Schiller JT, Quint W, Jimenez S, Safaeian M, Struijk L, Schussler J, Hildesheim A, Gonzalez P (2016) Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV infection. J Natl Cancer Inst 108 (1). https://doi.org/10.1093/jnci/djv302
Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, Brotherton JM (2012) Human papillomavirus vaccine introduction—the first five years. Vaccine 30 Suppl 5:F139–F148. https://doi.org/10.1016/j.vaccine.2012.05.039
Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert PH, Reisinger K, Haupt RM (2010) A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine 28(30):4719–4730. https://doi.org/10.1016/j.vaccine.2010.04.070
Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM (2011) Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 377(9783):2085–2092. https://doi.org/10.1016/s0140-6736(11)60551-5
Korostil IA, Ali H, Guy RJ, Donovan B, Law MG, Regan DG (2013) Near elimination of genital warts in Australia predicted with extension of human papillomavirus vaccination to males. Sex Transm Dis 40(11):833–835. https://doi.org/10.1097/olq.0000000000000030
Wangu Z, Hsu KK (2016) Impact of HPV vaccination on anogenital warts and respiratory papillomatosis. Hum Vaccin Immunother 12(6):1357–1362. https://doi.org/10.1080/21645515.2016.1172754
Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, Schwartz S, Castellsague X (2012) Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine 30 Suppl 5:F34–F54. https://doi.org/10.1016/j.vaccine.2012.05.070
San Giorgi MR, van den Heuvel ER, Tjon Pian Gi RE, Brunings JW, Chirila M, Friedrich G, Golusinski W, Graupp M, Horcasitas Pous RA, Ilmarinen T, Jackowska J, Koelmel JC, Ferran Vila F, Weichbold V, Wierzbicka M, Dikkers FG (2016) Age of onset of recurrent respiratory papillomatosis: a distribution analysis. Clin Otolaryngol 41(5):448–453. https://doi.org/10.1111/coa.12565
Novakovic D, Cheng ATL, Zurynski Y, Booy R, Walker PJ, Berkowitz R, Harrison H, Black R, Perry C, Vijayasekaran S, Wabnitz D, Burns H, Tabrizi SN, Garland SM, Elliott E, Brotherton JML (2017) A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J Infect Dis. https://doi.org/10.1093/infdis/jix498
Young DL, Moore MM, Halstead LA (2015) The use of the quadrivalent human papillomavirus vaccine (gardasil) as adjuvant therapy in the treatment of recurrent respiratory papilloma. J Voice: Off J Voice Found 29(2):223–229. https://doi.org/10.1016/j.jvoice.2014.08.003
Prue G, Lawler M, Baker P, Warnakulasuriya S (2017) Human papillomavirus (HPV): making the case for ‘Immunisation for All’. Oral Dis 23(6):726–730. https://doi.org/10.1111/odi.12562
Junor EJ, Kerr GR, Brewster DH (2010) Oropharyngeal cancer. Fastest increasing cancer in Scotland, especially in men. BMJ 340:c2512. https://doi.org/10.1136/bmj.c2512
Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB (2014) The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 151 (3):375–380. https://doi.org/10.1177/0194599814538605
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29(32):4294–4301. https://doi.org/10.1200/JCO.2011.36.4596
D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML (2007) Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356(19):1944–1956. https://doi.org/10.1056/NEJMoa065497
Gillison ML, Castellsague X, Chaturvedi A, Goodman MT, Snijders P, Tommasino M, Arbyn M, Franceschi S (2014) Eurogin roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer 134(3):497–507. https://doi.org/10.1002/ijc.28201
Du J, Nordfors C, Ahrlund-Richter A, Sobkowiak M, Romanitan M, Nasman A, Andersson S, Ramqvist T, Dalianis T (2012) Prevalence of oral human papillomavirus infection among youth. Swed Emerg Infecti Dis 18(9):1468–1471. https://doi.org/10.3201/eid1809.111731
Handisurya A, Schellenbacher C, Haitel A, Senger T, Kirnbauer R (2016) Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer 114(4):409–416. https://doi.org/10.1038/bjc.2015.462
Grun N, Ahrlund-Richter A, Franzen J, Mirzaie L, Marions L, Ramqvist T, Dalianis T (2015) Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up-study 2013–2014 after gradual introduction of public HPV vaccination. Inf Dis (London England) 47(1):57–61. https://doi.org/10.3109/00365548.2014.964764
Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, Jimenez S, Schiller JT, Lowy DR, van Doorn LJ, Wacholder S, Kreimer AR (2013) Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PloS One 8(7):e68329. https://doi.org/10.1371/journal.pone.0068329
VFC Program. Centers for disease control and prevention. https://www.cdc.gov/features/vfcprogram/. Accessed 12 Aug 2016
Takes RP, Wierzbicka M, D’Souza G, Jackowska J, Silver CE, Rodrigo JP, Dikkers FG, Olsen KD, Rinaldo A, Brakenhoff RH, Ferlito A (2015) HPV vaccination to prevent oropharyngeal carcinoma: What can be learned from anogenital vaccination programs? Oral oncology 51(12):1057–1060. https://doi.org/10.1016/j.oraloncology.2015.10.011
HPV Vaccine Coverage Maps-Inforgraphic (2016) Centers for disease control and prevention. https://www.cdc.gov/hpv/infographics/vacc-coverage.html. Accessed 12 Aug 2017
HPV Vaccine Uptake in Ireland: 2015/2016 (2017) Health service executive. https://www.hpsc.ie/a-z/vaccinepreventable/vaccination/immunisationuptakestatistics/hpvimmunisationuptakestatistics/File,16039,en.pdf. Accessed 12 Aug 2017
Bottiger M, Forsgren M (1997) Twenty years’ experience of rubella vaccination in Sweden: 10 years of selective vaccination (of 12-year-old girls and of women postpartum) and 13 years of a general two-dose vaccination. Vaccine 15(14):1538–1544
Bogaards JA, Wallinga J, Brakenhoff RH, Meijer CJ, Berkhof J (2015) Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. Bmj 350:h2016. https://doi.org/10.1136/bmj.h2016
Graham DM, Isaranuwatchai W, Habbous S, de Oliveira C, Liu G, Siu LL, Hoch JS (2015) A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer 121(11):1785–1792. https://doi.org/10.1002/cncr.29111
Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J (2013) Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer 13:10. https://doi.org/10.1186/1471-2407-13-10
Monsonego J, Breugelmans JG, Bouee S, Lafuma A, Benard S, Remy V (2007) [Anogenital warts incidence, medical management and costs in women consulting gynaecologists in France]. Gynecol Obstet Fertil 35(2):107–113. https://doi.org/10.1016/j.gyobfe.2006.12.010
Hartwig S, St Guily JL, Dominiak-Felden G, Alemany L, de Sanjose S (2017) Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect Agent Cancer 12:19. https://doi.org/10.1186/s13027-017-0129-6
Statistics by cancer type (2017) Cancer research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type. Accessed 15 Aug 2017
Funding
The authors have no funding or financial relationships to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Research involving human participants and animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hintze, J.M., O’Neill, J.P. Strengthening the case for gender-neutral and the nonavalent HPV vaccine. Eur Arch Otorhinolaryngol 275, 857–865 (2018). https://doi.org/10.1007/s00405-018-4866-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-4866-y