Abstract
Purpose
The impact of disease activity or treatments on health-related quality of life (HRQL) is crucial in Oncology, but adequate instruments for this assessment are scarce. Our aim is to validate the Mexican-Spanish version of the QLQ-EN24 questionnaire to evaluate HRQL in women with endometrial cancer (EC).
Methods
This is a prospective study of Mexican women with EC, attending a single cancer centre, who responded the QLQ-C30 and QLQ-EN24 instruments; usual psychometric analysis were performed as well as the association of HRQL scales and relevant clinical data. Correlation analysis was performed with the Spearman’s method, reliability analysis with the Cronbach’s alpha, known-group comparisons with the Kruskal–Wallis test, and survival analysis with the Kaplan–Meier method and Log-rank test.
Results
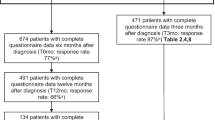
One hundred and eighty-nine women with EC were assessed. Most functional scales reported high values, and most symptom scales, low. Questionnaire compliance rates were high and internal consistency tests demonstrated adequate convergent and divergent validity. Cronbach’s α coefficients of the five multi-item scales the QLQ-EN24 instruments were from 0.659 to 0.887. Scales of the QLQ-C30 and QLQ-EN24 instruments distinguished among clinically distinct groups of patients, particularly based on serum albumin levels. The Urological symptoms, Gastrointestinal symptoms, Body image, Pelvic pain and Taste change scales were significantly associated with OS.
Conclusion
The Mexican-Spanish version of the QLQ-EN24 questionnaire is reliable and valid for the assessment of HRQL in patients with EC and can be broadly used in multi-national clinical trials. However, conclusions derived from scales evaluating sexual function should be handled carefully.

Similar content being viewed by others
Availability of data and material
The database in XLS format for this study is included in the supplementary material.
References
Ferlay J EM, Lam F, Colombet M, Mery L, Pieros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Cancer Research; c2018. Available from: https://gco.iarc.fr/today. (Accessed 17 Feb 2019)
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387:1094–1108
Ruvalcaba-Limon E, Cantu-de-Leon D, Leon-Rodriguez E, Cortes-Esteban P, Serrano-Olvera A, Morales-Vasquez F et al (2010) The first Mexican consensus of endometrial cancer. Grupo de investigacion en cancer de ovario y tumores ginecologicos de Mexico. Rev Invest Clin 62(583):5–605
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR (2006) Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a gynecologic oncology group study. Cancer 107:2786–2791
Group AES, Blake P, Swart AM, Orton J, Kitchener H, Whelan T, et al (2009) Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN5 randomised trials) pooled trial results, systematic review, and meta-analysis. Lancet 373:137–146
Salvesen HB, Haldorsen IS, Trovik J (2012) Markers for individualised therapy in endometrial carcinoma. Lancet Oncol 13:e353–e361
Angioli R, Plotti F, Cafa EV, Dugo N, Capriglione S, Terranova C et al (2013) Quality of life in patients with endometrial cancer treated with or without systematic lymphadenectomy. Eur J Obstet Gynecol Reprod Biol 170:539–543
Preston NJ, Wilson N, Wood NJ, Brine J, Ferreira J, Brearley SG (2015) Patient-reported outcome measures for use in gynaecological oncology: a systematic review. BJOG 122:615–622
Pisani C, Deantonio L, Surico D, Brambilla M, Galla A, Ferrara E et al (2016) Quality of life in patients treated by adjuvant radiotherapy for endometrial and cervical cancers: correlation with dose-volume parameters. Clin Transl Oncol 18:901–908
Peter M, Fayers DM (2016) Quality of life: the assessment, analysis and reporting of patient-reported outcomes, 3rd edn. Wiley-Blackwell, Chichester West
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Greimel E, Nordin A, Lanceley A, Creutzberg CL, van de Poll-Franse LV, Radisic VB et al (2011) Psychometric validation of the European Organisation for Research and Treatment of Cancer quality of life questionnaire-endometrial cancer module (EORTC QLQ-EN24). Eur J Cancer 47:183–190
Stukan M, Zalewski K, Mardas M, Filarska D, Szajewski M, Kmiec A et al (2018) Independent psychometric validation of European Organization for Research and treatment of cancer quality of life questionnaire-endometrial cancer module (EORTC QLQ-EN24). Eur J Cancer Care (Engl) 27:9
Lewin SN (2011) Revised FIGO staging system for endometrial cancer. Clin Obstet Gynecol 54:215–218
Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW et al (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88
Onate-Ocana LF, Alcantara-Pilar A, Vilar-Compte D, Garcia-Hubard G, Rojas-Castillo E, Alvarado-Aguilar S et al (2009) Validation of the Mexican Spanish version of the EORTC C30 and STO22 questionnaires for the evaluation of health-related quality of life in patients with gastric cancer. Ann Surg Oncol 16:88–95
Fayers PM AN, Bjordal K, Groenvold M, Curran D, Bottomley A. On behalf of the EORTC (2001) Quality of Life Group. Scoring procedures In: EORTC QLQ-C30 Scoring Manual Bruss. Eur J Cancer 38:125–133
Tabachnick BGFL (2019) Using multivariate statistics, 7th edn. California State University, Northridge
Thomas CR Jr (2016) The importance of quality of life assessment. JAMA Oncol 2:367–368
Salas-Vega S, Iliopoulos O, Mossialos E (2017) Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol 3:382–390
Quinten C, Martinelli F, Coens C, Sprangers MA, Ringash J, Gotay C et al (2014) A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 120:302–311
Oldenburg CS, Boll D, Nicolaije KA, Vos MC, Pijnenborg JM, Coebergh JW et al (2013) The relationship of body mass index with quality of life among endometrial cancer survivors: a study from the population-based PROFILES registry. Gynecol Oncol 129:216–221
van de Poll-Franse LV, Pijnenborg JM, Boll D, Voc MC, van den Berg H, Lybeert ML et al (2012) Health related quality of life and symptoms after pelvic lymphadenectomy or radiotherapy vs. no adjuvant regional treatment in early-stage endometrial carcinomaa large population-based study. Gynecol Oncol 127:153–160
Janda M, Gebski V, Brand A, Hogg R, Jobling TW, Land R et al (2010) Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol 11:772–780
Basch E, Geoghegan C, Coons SJ, Gnanasakthy A, Slagle AF, Papadopoulos EJ et al (2015) Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol 1:375–379
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ et al (2015) Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol 1:1051–1059
Acknowledgments
The authors sincerely thank the European Organization for Research and Treatment of Cancer, Quality of Life Group, particularly Ms. Maria Arnott, for her extensive support. We also thank Professor Deborah Aleman-Hoey, MD., for her English-language editorial review.
Funding
The INCan provided all funds for this study, and no external support has been received.
Author information
Authors and Affiliations
Contributions
DGR, ATL, ABG, ElMS, DDCH and LFOO: study concepts, literature review, protocol writing. DGR and LFOO: project development. GCAG, ARPM, EMS and DDCH: interviews, data acquisition, database construction. DGR, ATL, ABG, ElMS, EdMS, DDCH and LFOO: data management and quality control of the database. DGR, ATL, EdMS and LFOO: data analysis and interpretation. DGR, ATL, ABG, ElMS and LFOO: manuscript drafting. All authors: manuscript final review and approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no financial or non-financial conflict of interest.
Ethics approval
The “Comité de Investigación” and the “Comité de Ética en Investigación” (registration numbers 016/046/ICI and CEI1049, respectively) approved the research protocol.
Informed consent
Both committees belong to the “Instituto Nacional de Cancerología, México” and all patients signed an informed consent form.
Consent for publication
The original questionnaires in their European-Spanish versions were adapted and pilot tested, whilst these entire processes were supervised by EORTC. Both instruments were used with the permission of EORTC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gallardo-Rincón, D., Toledo-Leyva, A., Bahena-González, A. et al. Validation of the QLQ-EN24 instrument for the assessment of health-related quality of life for women with endometrial cancer in México. Arch Gynecol Obstet 304, 773–782 (2021). https://doi.org/10.1007/s00404-021-05990-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-05990-3