Abstract
Purpose
Mucinous ovarian carcinomas (MOCs) are relatively rare. It has been proposed that a subset of mucinous cystadenomas (MCAs) may progress to mucinous borderline tumors (MBTs), and then to MOCs. KRAS is the predominantly mutated gene in MOC; however, other associated mutations and the mechanism underlying carcinogenesis in MOC remain unclear. Here, we assessed molecular genetic alterations in mucinous ovarian tumors and constructed mutation profiles.
Methods
Using the Sanger sequencing method, we assessed genetic mutations (KRAS, BRAF, TP53, and PIK3CA) in 16 cases of MOC, 10 cases of MBT, and 12 cases of MCA.
Results
Among MOC cases, the prevalence of G12D and G13D KRAS mutations was 43.8% (7/16). No MOC cases showed V600E BRAF and TP53 mutations. Among MBT cases, the prevalence of G12D KRAS mutation was 20.0% (2/10), those of TP53 and PIK3CA mutations were nil, and that of V600E BRAF mutation was 40% (4/10). None of the genetic mutations assessed were detected among MCA cases.
Conclusion
These results suggest that MBT with V600E BRAF mutation may rarely progress to MOC, while MBT with G12D or G13D KRAS mutation may more commonly progress to MOC.
Similar content being viewed by others
Introduction
Ovarian cancer is the most lethal gynecological malignancy worldwide [1]; recently, its incidence has increased. A dualistic model has been proposed for epithelial ovarian cancer: low-grade disease (type I) develops in a stepwise manner from a benign cystadenoma to a borderline tumor, and then to a carcinoma, whereas high-grade disease (type II) develops de novo from the distal fallopian tube epithelium [2]. Mucinous ovarian tumors can be classified as type I tumors and mucinous ovarian carcinoma (MOC), which is a rare tumor that represents 2–4% of cases of epithelial ovarian carcinoma [3,4,5,6]. MOC has a good prognosis if diagnosed at an early stage; however, its prognosis is poor at advanced stages as it tends to be chemoresistant, particularly to platinum drugs [7].
Borderline tumors constitute approximately 10–20% of all epithelial ovarian masses [8]. The most common epithelial borderline tumor in Japan is the mucinous type, while the serous type is the most common in Western countries [9,10,11]. Ovarian borderline tumors are non-invasive cancers, have a good prognosis, and rarely require systemic therapy.
The RAS-RAF-MEK-ERK-MAP kinase pathway is often implicated in carcinogenesis; particularly, RAS oncogenes are key factors in tumor development [12]. BRAF and KRAS mutations are components of the mitogen-activated protein kinase (MAPK) cascade and KRAS mutations are common in mucinous ovarian tumors and prevalent among 40–50% of MOC cases [7]. It has been reported that the rates of KRAS mutations in normal ovaries, benign mucinous ovarian tumors, mucinous ovarian borderline tumors, and MOC are 0%, 57%, 90%, and 76%, respectively, suggesting that it may play a major role in the progression from benign tumors to carcinomas [13]. KRAS mutation leads to constitutive activation of the protein by increasing guanosine diphosphate/guanosine-5′-triphosphate exchange or by decreasing the guanosine triphosphatase activity of the protein, and thereby associates with constitutive activation of the epidermal growth factor receptor signaling pathway, and brings about increased cell proliferation [12, 14].
The three RAF genes (ARAF, BRAF, and CRAF) encode cytoplasmic serine/threonine kinases and are modulated by binding to RAS. BRAF mutations brings about ERK activation, which promotes the regulation of the G1/S transition of the cell cycle [12]. BRAF mutations were reported in a large proportion of cases of malignant melanoma [15], papillary thyroid cancer [16, 17], colon cancer [17, 18], and hairy cell leukemia [19] with poor outcomes. In contrast, they were reportedly associated with early-stage disease and improved outcomes in patients with low-grade serous ovarian cancer [20, 21]. Thus far, the role of BRAF mutations in mucinous ovarian carcinogenesis remains unclear. Additional mutations in mucinous tumors have been observed in TP53 and PIK3CA; however, all these cases emanated from Europe, Australia, or the United States [22,23,24,25,26,27,28]. Thus, the carcinogenesis of mucinous ovarian tumor among Japanese patients is still poorly understood. In the present study, we retrospectively investigated the mutation patterns of BRAF, KRAS, PIK3CA, and TP53 in mucinous cystadenomas (MCAs), mucinous borderline tumors (MBTs), and MOC to clarify the role of each gene in mucinous ovarian carcinogenesis.
Materials and methods
Tumor samples
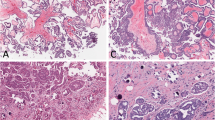
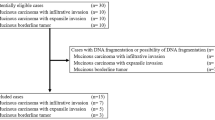
Formalin-fixed, paraffin-embedded tissue samples of 16 MOC, 10 MBT, and 12 MCA patients were used in this study. The samples were retrieved from the Department of Obstetrics and Gynecology, Shimane University Hospital (Izumo, Japan), which have collected from 2008 to 2017. The diagnoses were made based on conventional histopathologic examination of sections stained with hematoxylin and eosin. The tumors were categorized according to the World Health Organization subtype criteria by several pathologists in the Department of Pathology in Shimane University Hospital (Izumo, Japan). The tumors were staged according to the International Federation of Gynecology and Obstetrics classification system. All patients were primarily treated via surgery (i.e., total abdominal hysterectomy, bilateral salpingo-oophorectomy, and omentectomy) with or without pelvic and para-aortic lymph node dissection and adjuvant taxane/platinum combination chemotherapy. The resected specimens of each case were reviewed by a gynecological pathologist (N.I.) and a gynecologic oncologist (K.N.). The protocol for the acquisition of tissue specimens and clinical information was approved by the institutional review board of Shimane University Hospital (Approval No. 2004–0381). All participants provided written informed consent. The study was conducted in accordance with the tenets of the Declaration of Helsinki and Title 45 (United States Code of Federal Regulations), Part 46 (Protection of Human Subjects), effective December 13, 2001.
Microdissection and DNA extraction
Sixteen MOC, 10 MBT, and 12 MCA cases had sufficient tumor tissue for DNA extraction and sequence analysis. Tissue sections which were reviewed and marked with lines by a skillful gynecological pathologist were placed on membrane slides and counterstained with hematoxylin. Selected tumor tissues on 10-mm sections were dissected under a microscope using a 24-gauge needle to obtain a high percentage of tumor cells. After 48 h of digestion with a proteinase, DNA was extracted from the microdissected samples using a QIAmp DNA Micro Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. We have confirmed carcinoma/stroma ratio is more than 50% of each sample.
Direct sequence analysis
Polymerase chain reaction amplification was performed on exon two of KRAS, exon 15 of BRAF, exons 4–9 of TP53, and exons 9 and 20 of PIK3CA, using genomic DNA obtained from microdissected formalin-fixed, paraffin-embedded tissue using the following primers: forward 5′-TTAACCTTATGTGTGACATGTTCTAA-3′, reverse 5′-AGAATGGTCCTGCACCAGTAA-3′ for exon two of KRAS; forward 5′-TGCTTGCTCTGATAGGAAAATG-3′, reverse 5′-AGCATCTCAGGGCCAAAAAT-3′ for exon 15 of BRAF; forward 5′-CCTGGTCCTCTGACTGCTCT-3′, reverse 5′-GCCAGGCATTGAAGTCTCAT-3′ for exon 4 of TP53; forward 5′-TCAGATAGCGATGGTGAGCA-3′, reverse 5′-CTTAACCCCTCCTCCCAGAG-3′ for exon five of TP53; forward 5′-TCTGTCTCCTTCCTCTTCCTACA-3′, reverse 5′-AACCAGCCCTGTCGTCTCT-3′ for exon 6 of TP53; forward 5′-CTTGGGCCTGTGTTATCTCC-3′, reverse 5′-GGGTCAGAGGCAAGCAGA-3′ for exon seven of TP53; forward 5′-GGGAGTAGATGGAGCCTGGT-3′, reverse 5′-GCTTCTTGTCCTGCTTGCTT-3′ for exon 8 of TP53; forward 5′-GGAGACCAAGGGTGCAGTTA-3′, reverse 5′-CCCCAATTGCAGGTAAAACA-3′ for exon nine of TP53; forward 5′-GGAAAAATATGACAAAGAAAGC-3′, reverse 5′-CTGAGATCAGCCAAATTCAGTT-3′ for exon nine of PIK3CA; and forward 5′-CTCAATGATGCTTGGCTCTG-3′, reverse 5′-TGGAATCCAGAGTGAGCTTTC-3′ for exon 20 of PIK3CA. All polymerase chain reaction-amplified products were sequenced at Beckman Coulter (Danvers, MA, USA) and analyzed with the Mutation Surveyor DNA Variant Analysis Software (Tokyo, Japan).
Statistical analysis
All results are expressed as means ± standard deviations. In some cases, the three groups were compared using the chi-square test and the Tukey–Kramer test, as appropriate. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Japan). All differences in analysis items were considered significant at p < 0.05.
Results
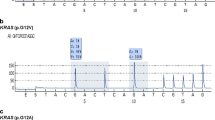
To assess the mutation profiles of mucinous tumors of the ovary, we performed direct sequence analysis on 38 tumor specimens, including 16 MOCs, 10 MBTs, and 12 MCAs. The clinical characteristics of the patients are summarized in (Table 1). The mean ages of the patients at diagnosis were 59.6 ± 16.3 years for MOC, 56.5 ± 20.0 years for MBT, and 58.0 ± 18.2 years for MCA. There were no significant differences in the characteristics of the participants, cancer antigen (CA) 125 level, and CA19-9 level. A majority of the patients (81.3%) with MOC were found to have early stage I or II disease at diagnosis, and only 18.8% presented with advanced stage III or IV disease. Figure 1 shows representative examples of the histological appearance of mucinous ovarian tumors. Figure 2 shows typical point mutations in KRAS and BRAF.
All 38 cases were assessed for mutations in the KRAS, BRAF, TP53, and PIK3CA genes. KRAS mutations were detected in 7 of the 16 (43.8%) MOC cases and in 2 of the 10 (20%) MBT cases (Table 2). However, no KRAS mutations were detected in MCA cases. KRAS mutations tended to occur more frequently in MBT than in MCA (p = 0.066, Chi square test). BRAF mutations in exon 15 were only detected in four of the MBT cases, but not in the MOC or MCA cases. None of the mucinous tumor specimens showed TP53 mutations. BRAF mutations occurred significantly more frequently in MBT cases than in MOC cases (*p = 0.042, Chi square test) (Table 3). PIK3CA mutation was detected in only one case of MCA.
Discussion
In the present study, we performed direct sequence analysis on 38 tumors, including 16 MOC, 10 MBT, and 12 MCA specimens to elucidate the genetic profile of mucinous tumors of the ovary. Interestingly, BRAF mutations were more common in MBT than in MOC. However, KRAS mutations occurred with high frequency in MOC but with low frequency in MBT. No mutations were detected in the analyzed genes of MCA. These findings indicated that, in the disease continuum from MBT to MOC, the BRAF mutation in MBT may not result in progression to MOC, while KRAS mutations in MBT may be associated with progression to MOC (Fig. 3).
BRAF is a meaningful serine/threonine kinase that is an element of the RAS-RAF-MEK-ERK signaling pathway and plays a key role in cell proliferation and apoptosis. The complexity of this pathway is increased due to the multiplicity of its components. There are three RAS (HRAS, NRAS, and KRAS), three RAF (ARAF, BRAF, and CRAF), two MEK (MEK1 and MEK2), and two ERK (ERK1 and ERK2) genes. They encode proteins and do not have redundant functions [29]. BRAF binds to CRAF and activates its transphosphorylation, thereby regulating the pathway subtly [29].
The V600E BRAF mutation constitutes over 90% of all BRAF mutations in melanoma [29]. It has been found to activate the MAPK pathway by activating mutations of either NRAS or BRAF in most melanomas [30]. The BRAF and CRAF protein kinases are the most critical mediators of activated RAS [31]. For mutated NRAS, CRAF seems to be important in the downstream activation of MAPKs [32, 33]. RAF interacts with MEK and phosphorylates it, thereby activating ERK [31, 34, 35]. Activated ERK promotes the signal, through altered transcription of several genes [36]. BRAF mutations are observed in most melanocytic nevi (70–80%), metastatic melanomas (40–50%), and vertical growth phase melanomas (40–50%) [37,38,39], and might be an acquired event in early invasive melanoma that induces clonal expansion and tumor progression [36]. Consequently, BRAF mutation is associated with poor prognosis in not only melanoma but also papillary thyroid cancer and metastatic colon cancer [15,16,17,18]. In contrast, BRAF mutations were present in MBT but not in MOC in this study, suggesting that BRAF mutations are associated with the indolent type of MBT. Wong et al. reported that BRAF mutations are infrequent in advanced-stage low-grade serous ovarian carcinomas and could be improved prognostic markers [20]. Grisham et al. demonstrated that the presence of BRAF mutations in serous borderline ovarian tumor or low-grade serous ovarian carcinoma was relevant to early-stage disease and favorable prognoses [21]. Recently, it has been reported that lack of Cdkn2a in V600E BRAF mutated melanocytes in rodents is associated with rare progression to melanoma [40]. In MOC, Cdkn2a/b homozygous deletions/mutations were detected at high frequencies [41]. From these reports, it appears that loss of Cdkn2a in mucinous ovarian tumors with V600E BRAF mutation impairs progression to carcinoma. Therefore, BRAF mutation is associated with early-stage disease, such as MBT, and was not detected in MOC in the present study.
KRAS is the predominant mutated gene in MOC and may be related to the progression from benign to malignant tumors [7]. It has been reported that CRAF is a best target for carcinoma with KRAS mutations and intensifies MAPK signaling [42, 43]. Our results are consistent with those of previous studies regarding KRAS; the prevalences of KRAS mutations were 0%, 20%, and 43% among MCA, MBT, and MOC specimens, respectively. We also found that some cases had both KRAS and BRAF mutations in MBT. These MBT cases with both KRAS and BRAF mutations might progress to MOC earlier than would those without these mutations.
Recently, it has been reported that TP53 mutations were key drivers of progression from MBT to MOC [44]. Surprisingly, in the present study, this mutation was not detected in all mucinous ovarian tumors. This discrepancy may have occurred because we investigated only mucinous ovarian tumor specimens obtained from Japanese patients. The carcinogenesis of MOC may be affected by ethnic genetic background. On the other hand, PCR amplification was not performed on exon 2, 3, 10 and 11. There is a possibility that TP53 mutations could be detected in these exons. Additionally, some MOC cases are high-grade features and they may have TP53 mutations without exon 4–9.
Our study indicates that BRAF and KRAS mutations are useful as prognostic biomarkers in MBT patients undergoing surgery. Single BRAF mutations in MBT may predict a favorable outcome. However, the patients with KRAS mutations might progress to MOC and require careful long-term follow-up.
The present study has several limitations. First, the number of samples in this study is small. This study is ongoing and the number of samples will increase. This will enable us to investigate statistically the relationship between the mutations identified in the present study and patient outcomes. Second, we did not search for loss or mutation of Cdkn2a in the present study. In addition, we also need to study CRAF mutations in mucinous ovarian tumors. Last, we assessed genetic mutations via Sanger sequencing; therefore, the kinds of gene mutations assessed were limited. Further experimentation with next generation sequencing is necessary to determine details of the molecular mechanism underlying mucinous ovarian carcinogenesis.
In summary, V600E BRAF mutations were detected only in MBT, while G12D/G13D KRAS mutations were detected more commonly in MOC than in MBT. We posit that MBT with V600E BRAF mutation may not progress to MOC and predict a favorable outcome, while MBT with G12D/G13D KRAS mutation may progress to MOC in the future.
Abbreviations
- MOC:
-
Mucinous ovarian carcinoma
- MBT:
-
Mucinous ovarian tumor
- MCA:
-
Mucinous cystadenoma
References
Siegel RL, Miller KD (2019) Cancer statistics. CA Cancer J Clin 69(1):7–34. https://doi.org/10.3322/caac.21551
Shih Ie M, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164(5):1511–1518
Gilks CB (2004) Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol 23(3):200–205
Kurman RJ, Shih Ie M (2011) Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol 42(7):918–931. https://doi.org/10.1016/j.humpath.2011.03.003
Tabrizi AD, Kalloger SE, Kobel M, Cipollone J, Roskelley CD, Mehl E, Gilks CB (2010) Primary ovarian mucinous carcinoma of intestinal type: significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Pathol 29(2):99–107. https://doi.org/10.1097/PGP.0b013e3181bbbcc1
Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM (2004) The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 23(1):41–44. https://doi.org/10.1097/01.pgp.0000101080.35393.16
Ricci F, Affatato R, Carrassa L, Damia G (2018) Recent insights into mucinous ovarian carcinoma. Int J Mol Sci 19:6. https://doi.org/10.3390/ijms19061569
Vang R, Shih Ie M, Kurman RJ (2009) Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 16(5):267–282. https://doi.org/10.1097/PAP.0b013e3181b4fffa
Mandai M, Konishi I, Kuroda H, Komatsu T, Yamamoto S, Nanbu K, Matsushita K, Fukumoto M, Yamabe H, Mori T (1998) Heterogeneous distribution of K-ras-mutated epithelia in mucinous ovarian tumors with special reference to histopathology. Hum Pathol 29(1):34–40
Takeshima Y, Amatya VJ, Daimaru Y, Nakayori F, Nakano T, Inai K (2001) Heterogeneous genetic alterations in ovarian mucinous tumors: application and usefulness of laser capture microdissection. Hum Pathol 32(11):1203–1208. https://doi.org/10.1053/hupa.2001.28956
Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao SW (1993) Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res 53(7):1489–1492
Mayr D, Hirschmann A, Lohrs U, Diebold J (2006) KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol 103(3):883–887. https://doi.org/10.1016/j.ygyno.2006.05.029
Lee YJ, Lee MY, Ruan A, Chen CK, Liu HP, Wang CJ, Chao WR, Han CP (2016) Multipoint Kras oncogene mutations potentially indicate mucinous carcinoma on the entire spectrum of mucinous ovarian neoplasms. Oncotarget 7(50):82097–82103. https://doi.org/10.18632/oncotarget.13449
Fabjani G, Kriegshaeuser G, Schuetz A, Prix L, Zeillinger R (2005) Biochip for K-ras mutation screening in ovarian cancer. Clin Chem 51(4):784–787. https://doi.org/10.1373/clinchem.2004.041194
Dhomen N, Reis-Filho JS, da Rocha DS, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15(4):294–303. https://doi.org/10.1016/j.ccr.2009.02.022
Xing M (2007) BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28(7):742–762. https://doi.org/10.1210/er.2007-0007
Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P (2014) Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology 46(6):509–517. https://doi.org/10.1097/PAT.0000000000000119
Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouche O, Tabernero J, Mini E, Goldberg RM, Folprecht G, Luc Van Laethem J, Sargent DJ, Alberts SR, Emile JF, Laurent Puig P, Sinicrope FA (2017) Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 109:5. https://doi.org/10.1093/jnci/djw272
Kreitman RJ (2019) Hairy cell leukemia: present and future directions. Leuk Lymphoma 1:11. https://doi.org/10.1080/10428194.2019.1608536
Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, Malpica A, Wolf JK, Lu KH, Gershenson DM (2010) BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 177(4):1611–1617. https://doi.org/10.2353/ajpath.2010.100212
Grisham RN, Iyer G, Garg K, Delair D, Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, Levine DA, Aghajanian C, Solit DB (2013) BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 119(3):548–554. https://doi.org/10.1002/cncr.27782
Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J (1997) K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer 79(8):1581–1586
Hunter SM, Gorringe KL, Christie M, Rowley SM, Bowtell DD (2012) Australian Ovarian Cancer Study G, Campbell IG Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin Cancer Res 18(19):5267–5277. https://doi.org/10.1158/1078-0432.CCR-12-1103
Ryland GL, Hunter SM, Doyle MA, Rowley SM, Christie M, Allan PE, Bowtell DD (2013) Australian Ovarian Cancer Study G, Gorringe KL, Campbell IG RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol. 229(3):469–476. https://doi.org/10.1002/path.4134
Wang Y, Helland A, Holm R, Kristensen GB, Borresen-Dale AL (2005) PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat 25(3):322. https://doi.org/10.1002/humu.9316
Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, Halling K, Karnezis A, Senz J, Yang W, Prigge ES, Reuschenbach M, Doeberitz MV, Gilks BC, Huntsman DG, Bakkum-Gamez J, McAlpine JN, Anglesio MS (2015) Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer 15:415. https://doi.org/10.1186/s12885-015-1421-8
Ryland GL, Hunter SM, Doyle MA, Caramia F, Li J, Rowley SM, Christie M, Allan PE, Stephens AN, Bowtell DD (2015) Australian Ovarian Cancer Study G, Campbell IG, Gorringe KL Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med 7(1):87. https://doi.org/10.1186/s13073-015-0210-y
Vereczkey I, Serester O, Dobos J, Gallai M, Szakacs O, Szentirmay Z, Toth E (2011) Molecular characterization of 103 ovarian serous and mucinous tumors. Pathol Oncol Res 17(3):551–559. https://doi.org/10.1007/s12253-010-9345-8
Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R (2010) Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140(2):209–221. https://doi.org/10.1016/j.cell.2009.12.040
Omholt K, Platz A, Kanter L, Ringborg U, Hansson J (2003) NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 9(17):6483–6488
Beeram M, Patnaik A, Rowinsky EK (2005) Raf: a strategic target for therapeutic development against cancer. J Clin Oncol 23(27):6771–6790. https://doi.org/10.1200/JCO.2005.08.036
Marquette A, Andre J, Bagot M, Bensussan A, Dumaz N (2011) ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat Struct Mol Biol 18(5):584–591. https://doi.org/10.1038/nsmb.2022
Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, Bastian BC, Springer C, Marais R (2006) In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66(19):9483–9491. https://doi.org/10.1158/0008-5472.CAN-05-4227
Garnett MJ, Rana S, Paterson H, Barford D, Marais R (2005) Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell 20(6):963–969. https://doi.org/10.1016/j.molcel.2005.10.022
Terai K, Matsuda M (2006) The amino-terminal B-Raf-specific region mediates calcium-dependent homo- and hetero-dimerization of Raf. EMBO J 25(15):3556–3564. https://doi.org/10.1038/sj.emboj.7601241
Sullivan RJ, Flaherty K (2013) MAP kinase signaling and inhibition in melanoma. Oncogene 32(19):2373–2379. https://doi.org/10.1038/onc.2012.345
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954. https://doi.org/10.1038/nature00766
Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS (2003) High frequency of BRAF mutations in nevi. Nat Genet 33(1):19–20. https://doi.org/10.1038/ng1054
Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, Aaronson SA (2003) BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res 63(14):3883–3885
Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, Erdelyi I, Platt JT, Huang L, Theodosakis N, Zaidi MR, Tighe S, Davies MA, Dankort D, McMahon M, Merlino G, Bardeesy N, Bosenberg M (2015) mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formation. Cancer Cell 27(1):41–56. https://doi.org/10.1016/j.ccell.2014.11.014
Mueller JJ, Schlappe BA, Kumar R, Olvera N, Dao F, Abu-Rustum N, Aghajanian C, DeLair D, Hussein YR, Soslow RA, Levine DA, Weigelt B (2018) Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol Oncol 150(1):127–135. https://doi.org/10.1016/j.ygyno.2018.05.008
Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR (2018) Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 37(24):3183–3199. https://doi.org/10.1038/s41388-018-0171-x
McCormick F (2018) c-Raf in KRas mutant cancers: a moving target. Cancer Cell 33(2):158–159. https://doi.org/10.1016/j.ccell.2018.01.017
Cheasley D, Wakefield MJ, Ryland GL, Allan PE, Alsop K, Amarasinghe KC, Ananda S, Anglesio MS, Au-Yeung G, Bohm M, Bowtell DDL, Brand A, Chenevix-Trench G, Christie M, Chiew YE, Churchman M, DeFazio A, Demeo R, Dudley R, Fairweather N, Fedele CG, Fereday S, Fox SB, Gilks CB, Gourley C, Hacker NF, Hadley AM, Hendley J, Ho GY, Hughes S, Hunstman DG, Hunter SM, Jobling TW, Kalli KR, Kaufmann SH, Kennedy CJ, Kobel M, Le Page C, Li J, Lupat R, McNally OM, McAlpine JN, Mes-Masson AM, Mileshkin L, Provencher DM, Pyman J, Rahimi K, Rowley SM, Salazar C, Samimi G, Saunders H, Semple T, Sharma R, Sharpe AJ, Stephens AN, Thio N, Torres MC, Traficante N, Xing Z, Zethoven M, Antill YC, Scott CL, Campbell IG, Gorringe KL (2019) The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun 10(1):3935. https://doi.org/10.1038/s41467-019-11862-x
Funding
This work was supported by JSPS KAKENHI grant numbers 18K09229 and 18K09291.
Author information
Authors and Affiliations
Contributions
KO and KN drafted the manuscript. KO, TI, MI, KN, TM, HY, KI, NI, and RS carried out the molecular genetic studies. KO carried out the statistical analyses. KN participated in the design of the study. SK conceived of the study, participated in its design and coordination, and helped in drafting the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohnishi, K., Nakayama, K., Ishikawa, M. et al. Mucinous borderline ovarian tumors with BRAFV600E mutation may have low risk for progression to invasive carcinomas. Arch Gynecol Obstet 302, 487–495 (2020). https://doi.org/10.1007/s00404-020-05638-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05638-8