Abstract
Purpose
To assess whether there are proteins in endometrial fluid aspirate (EFA) that predict implantation.
Methods
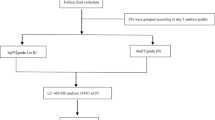
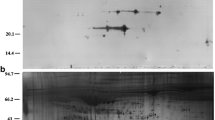
The population under study consisted of 285 women undergoing embryo transfer (ET). Endometrial fluid aspiration was performed immediately before ET. Results of proteomic analysis of EFA were compared between 33 cases who achieved pregnancy and 33 who did not. Samples were analysed by 2D electrophoresis and mass spectrometry. Blood samples were studied by ELISA Pregnancy rates and maternal complications were compared to those in women refusing aspiration.
Results
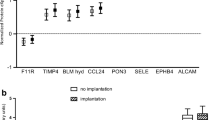
We found 23 proteins differentially expressed in the EFA in conception cycles: 4 up-regulated proteins and 19 down-regulated (FC = 0.31 0.78) (among others, arginase-1, actin B, PARK-7, cofilin-1, stathmin, annexin-2 and CAPZB). Among the five studied proteins that were differentially expressed in EFA, none was differentially expressed in serum. The aspiration procedure had no impact on pregnancy rate. No maternal complications were reported.
Conclusions
We found a very different protein profile in implantative cycles, the majority of proteins being down-regulated. This probably reflects a different endometrial functional status, more favourable to implantation. EFA proteomic analysis could be a useful tool in the planning ET strategies.


Similar content being viewed by others
References
Matorras R, Urquijo E, Mendoza R, Corcóstegui B, Expósito A, Rodríguez-Escudero FJ (2002) Ultrasound-guided embryo transfer improves pregnancy rates and increases the frequency of easy transfers. Hum Reprod 17:1762–1766
Friedler S, Schenker JG, Herman A, Lewin A (1996) The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum Reprod Update 2:323–335
Valdez-Morales FJ, Gamboa-Domínguez A, Vital-Reyes VS, Cruz JC, Chimal-Monroy J, Franco-Murillo Y, Cerbón M (2015) Changes in receptivity epithelial cell markers of endometrium after ovarian stimulation treatments: its role during implantation window. Reprod Health 12:45–56
Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL (2009) Proteomic analysis of the luteal endometrial secretome. Reprod Sci 16:883–893
Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, Matorras R, Prieto B, González S, Nagore D, Simón L, Elortza F (2009) Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res 8:4622–4632
Ametzazurra A, Matorras R, García-Velasco JA, Prieto B, Simón L, Martínez A, Nagore D (2009) Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum Reprod 24:954–965
Edgell TA, Rombauts LJ, Salamonsen LA (2013) Assessing receptivity in the endometrium: the need for a rapid, non-invasive test. Reprod Biomed Online 27:486–496
Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE (2001) Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 64:1608–1613
Dunlap KA, Filant J, Hayashi K, Rucker EB 3rd, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE (2011) Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 85:386–396
Garrido-Gómez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C (2013) Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril 99:1078–1085
Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, Garrido N, Pellicer A, Simón C (2013) The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril 99:508–517
Vilella F, Ramirez LB, Simón C (2013) Lipidomics as an emerging tool to predict endometrial receptivity. Fertil Steril 99:1100–1106
Bourgain C, Devroey P (2003) The endometrium in stimulated cycles for IVF. Hum Reprod Update 9:515–522
Kolibianakis EM, Devroey P (2002) The luteal phase after ovarian stimulation. Reprod Biomed Online 5:26–35
Noyes RH, Hertig AT, Rock J (1950) Dating the endometrial biopsy. Fertil Steril 1:3–25
Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S (2012) Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod Biomed Online 24:23–34
van der Gaast MH, Beier-Hellwig K, Fauser BC, Beier HM, Macklon NS (2003) Endometrial secretion aspiration prior to embryo transfer does not reduce implantation rates. Reprod Biomed Online 7:105–109
Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, Gutknecht D, Fauser BJ, Heijnen CJ, Macklon NS (2009) Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod Biomed Online 18:85–94
Chen JI, Hannan NJ, Mak Y, Nicholls PK, Zhang J, Rainczuk A, Stanton PG, Robertson DM, Salamonsen LA, Stephens AN (2009) Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res 8:2032–2044
DeSouza L, Diehl G, Yang EC, Guo J, Rodrigues MJ, Romaschin AD, Colgan TJ, Siu KW (2005) Proteomic analysis of the proliferative and secretory phases of the human endometrium: protein identification and differential protein expression. Proteomics 5:270–328
Parmar T, Gadkar-Sable S, Savardekar L, Katkam R, Dharma S, Meherji P, Puri CP, Sachdeva G (2009) Protein profiling of human endometrial tissues in the midsecretory and proliferative phases of the menstrual cycle. Fertil Steril 92:1091–1103
Li J, Tan Z, Li MT, Liu YL, Liu Q, Gu XF, Zhou JZ, Zhuang GL (2006) Study of altered expression of annexin IV and human endometrial receptivity. Zhonghua Fu Chan KeZaZhi. 41:803–805
Domínguez F, Garrido-Gómez T, López JA, Camafeita E, Quiñonero A, Pellicer A, Simón C (2009) Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod 24:2607–2617
Forde N, McGettigan PA, Mehta JP, O’Hara L, Mamo S, Bazer FW, Spencer TE, Lonergan P (2014) Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 147:575–587
Yap J, Foo CF, Lee MY, Stanton PG, Dimitriadis E (2011) Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. Reprod Biol Endocrinol 9:73–87
Tressler RJ, Updyke TV, Yeatman T, Nicolson GL (1993) Extracellular annexin II is associated with divalent cation-dependent tumor cell-endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. J Cell Biochem 53:265–276
Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V (2008) Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci 121:2177–2185
Wolberg AS, Roubey RA (2005) Annexin A2: better left alone. Blood 105:1845–1846
Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S (2005) In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 20:2104–2117
Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M (2001) Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Steril 75:361–366
Funding
This study was partially funded by a Grant for Fertility Innovation (GFI, 2011) from Merck, Darmstadt, Germany.
Author information
Authors and Affiliations
Contributions
RM: manuscript writing, supervision. SQ: data collection. BC: data collection. BP: supervision. AE: investigation, manuscript writing. RM: protocol. AR: supervision. DM: data collection. MF: protocol. FE: methodology. AA: methodology, validation, software. DN: methodology, validation, software
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have not conflict of interest.
Informed consent
We obtained approval from the Institutional Review Board (CEIC 09/54 and CEIC 11/45) and informed consent from participants.
Rights and permissions
About this article
Cite this article
Matorras, R., Quevedo, S., Corral, B. et al. Proteomic pattern of implantative human endometrial fluid in in vitro fertilization cycles. Arch Gynecol Obstet 297, 1577–1586 (2018). https://doi.org/10.1007/s00404-018-4753-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4753-1