Abstract
Purpose
No research has studied the effect of GH co-treatment in mild stimulation protocol for poor responders. We therefore conducted this retrospective analysis to assess the outcome of IVF/ICSI cycles after the adjunct GH use to the mild stimulation protocol in poor responders.
Methods
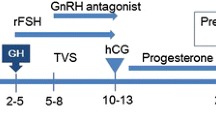
132 poor responders who received mild stimulation protocol at Reproductive Medicine Center of Changzheng Hospital from January 2014 to December 2016 were included in this study. Good-quality embryo rate, clinical pregnancy rate, and live birth rate were compared between the GH group (n = 61) and control group (n = 71).
Results
IVF good-quality embryo rate (68.1 versus 51.5%; P = 0.008*) and ICSI good-quality embryo rate (53.9 versus 36.7%; P = 0.045*) was significantly higher in the GH group. Though the clinical outcomes did not reach a statistically significant difference between the two groups due to the limited sample size, there was a trend of higher rate in GH group in the aspect of clinical pregnancy rate (52.4 versus 47.1%; P = 0.609) and live birth rate (35.7 versus 27.5%; P = 0.392).
Conclusion
The results suggested that the adjuvant GH treatment in mild stimulation protocol for poor responders could significantly improve good-quality embryo rate, and might therefore improve the clinical outcomes.
Similar content being viewed by others
References
Oudendijk JF, Yarde F, Eijkemans MJ, Broekmans FJ, Broer SL (2012) The poor responder in IVF: is the prognosis always poor?: a systematic review. Hum Reprod Updat 18(1):1–11
Biljan MM, Buckett WM, Dean N, Phillips SJ, Tan SL (2000) The outcome of IVF-embryo transfer treatment in patients who develop three follicles or less. Hum Reprod 15(10):2140–2144
De Sutter P, Dhont M (2003) Poor response after hormonal stimulation for in vitro fertilization is not related to ovarian aging. Fertil Steril 79(6):1294–1298
Galey-Fontaine J, Cedrin-Durnerin I, Chaibi R, Massin N, Hugues JN (2005) Age and ovarian reserve are distinct predictive factors of cycle outcome in low responders. Reprod Biomed Online 10(1):94–99
Baka S, Makrakis E, Tzanakaki D et al (2006) Poor responders in IVF: cancellation of a first cycle is not predictive of a subsequent failure. Ann N Y Acad Sci 1092:418–425
Saldeen P, Kallen K, Sundstrom P (2007) The probability of successful IVF outcome after poor ovarian response. Acta Obstet Gynecol Scand 86(4):457–461
Hendriks DJ, te Velde ER, Looman CW, Bancsi LF, Broekmans FJ (2008) Expected poor ovarian response in predicting cumulative pregnancy rates: a powerful tool. Reprod Biomed Online 17(5):727–736
Zhen XM, Qiao J, Li R, Wang LN, Liu P (2008) The clinical analysis of poor ovarian response in in vitro-fertilization embryo-transfer among Chinese couples. J Assist Reprod Genet 25(1):17–22
Ebrahimi M, Akbari-Asbagh F, Ghalandar-Attar M (2017) Letrozole + GnRH antagonist stimulation protocol in poor ovarian responders undergoing intracytoplasmic sperm injection cycles: an RCT. Int J Reprod Biomed (Yazd) 15(2):101–108
Youssef MA, van Wely M, Al-Inany H et al (2017) A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: a multicenter randomized non-inferiority trial. Hum Reprod 32(1):112–118
Bassiouny YA, Dakhly DM, Bayoumi YA, Hashish NM (2016) Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril 105(3):697–702
Bayoumi YA, Dakhly DM, Bassiouny YA, Hashish NM (2015) Addition of growth hormone to the microflare stimulation protocol among women with poor ovarian response. Int J Gynaecol Obstet 131(3):305–308
Merviel P, Cabry-Goubet R, Lourdel E et al (2015) Comparative prospective study of 2 ovarian stimulation protocols in poor responders: effect on implantation rate and ongoing pregnancy. Reprod Health 12:52
Davar R, Oskouian H, Ahmadi S, Firouzabadi RD (2010) GnRH antagonist/letrozole versus microdose GnRH agonist flare protocol in poor responders undergoing in vitro fertilization. Taiwan J Obstet Gynecol 49(3):297–301
Yarali H, Esinler I, Polat M, Bozdag G, Tiras B (2009) Antagonist/letrozole protocol in poor ovarian responders for intracytoplasmic sperm injection: a comparative study with the microdose flare-up protocol. Fertil Steril 92(1):231–235
Eftekhar M, Mohammadian F, Davar R, Pourmasumi S (2014) Comparison of pregnancy outcome after letrozole versus clomiphene treatment for mild ovarian stimulation protocol in poor responders. Iran J Reprod Med 12(11):725–730
Oktem M, Guler I, Erdem M, Erdem A, Bozkurt N, Karabacak O (2015) Comparison of The effectiveness of clomiphene citrate versus letrozole in mild IVF in poor prognosis subfertile women with failed IVF cycles. Int J Fertil Steril 9(3):285–291
Ferraretti AP, Gianaroli L, Magli MC, Devroey P (2015) Mild ovarian stimulation with clomiphene citrate launch is a realistic option for in vitro fertilization. Fertil Steril 104(2):333–338
Bachelot A, Monget P, Imbert-Bollore P et al (2002) Growth hormone is required for ovarian follicular growth. Endocrinology 143(10):4104–4112
Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G (2009) Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update 15(6):613–622
Lattes K, Brassesco M, Gomez M, Checa MA (2015) Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecol Endocrinol 31(7):565–568
Zhuang GL, Wong SX, Zhou CQ (1994) The effect of co-administration of low dosage growth hormone and gonadotropin for ovarian hyperstimulation in vitro fertilization and embryo transfer. Zhonghua Fu Chan Ke Za Zhi 29(8):471–474, 510
Suikkari A, MacLachlan V, Koistinen R, Seppala M, Healy D (1996) Double-blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertil Steril 65(4):800–805
Owen EJ, Shoham Z, Mason BA, Ostergaard H, Jacobs HS (1991) Cotreatment with growth hormone, after pituitary suppression, for ovarian stimulation in in vitro fertilization: a randomized, double-blind, placebo-control trial. Fertil Steril 56(6):1104–1110
Hart RJ, Rombauts L, Norman RJ (2017) Growth hormone in IVF cycles: any hope? Curr Opin Obstet Gynecol 29(3):119–125
Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC (2009) How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril 91(3):749–766
Li XL, Wang L, Lv F et al (2017) The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: a systematic review and meta-analysis. Medicine (Baltimore) 96(12):e6443
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L (2011) ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7):1616–1624
Weigert M, Krischker U, Pohl M, Poschalko G, Kindermann C, Feichtinger W (2002) Comparison of stimulation with clomiphene citrate in combination with recombinant follicle-stimulating hormone and recombinant luteinizing hormone to stimulation with a gonadotropin-releasing hormone agonist protocol: a prospective, randomized study. Fertil Steril 78(1):34–39
Baart EB, Martini E, Eijkemans MJ et al (2007) Milder ovarian stimulation for in vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod 22(4):980–988
Hohmann FP, Macklon NS, Fauser BC (2003) A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab 88(1):166–173
Yoshimura Y, Ando M, Nagamatsu S et al (1996) Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol Reprod 55(1):152–160
Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJ (1994) Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology 135(5):1845–1853
Baker J, Hardy MP, Zhou J et al (1996) Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10(7):903–918
Zhou J, Kumar TR, Matzuk MM, Bondy C (1997) Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol 11(13):1924–1933
Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A (2010) Growth hormone for in vitro fertilization. Cochrane Database Syst Rev 1:Cd000099
Acknowledgements
We thank the patients for participating in this study.
Funding
This study was supported by the Science and Technology Commission of Shanghai Municipality [Grant Numbers 16DZ0500402, 1641963500].
Author information
Authors and Affiliations
Contributions
WL conceived and designed the experiments. KC and WP performed the experiments and analyzed the data. KC and WL wrote the main manuscript. NS and QZ prepared the tables and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chu, K., Pang, W., Sun, N. et al. Outcomes of poor responders following growth hormone co-treatment with IVF/ICSI mild stimulation protocol: a retrospective cohort study. Arch Gynecol Obstet 297, 1317–1321 (2018). https://doi.org/10.1007/s00404-018-4725-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4725-5