Abstract
Purpose
Recent studies showed differences in the risk of venous thrombosis between different combined hormonal contraceptives. Database studies comprising large cohorts can add relevant aspects from daily clinical practice. The purpose of this study was to evaluate different progestogen in combination with ethinylestradiol on the risk of venous thrombosis in Germany.
Methods
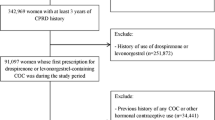
Computerized data from 68,168 contraceptive users in gynecological practices throughout Germany (Disease Analyzer Database) were analyzed. The adjusted odds ratios for risk of thrombosis were estimated in users of different oral contraceptive (OC) formulations relative to users of levonorgestrel-containing preparations.
Results
In total, 38 (0.06 %) of the 68,168 contraceptive users had a recorded diagnosis of thrombosis within 365 days after the initial prescription. The adjusted risk was 1.95 for desogestrel (95 % CI 0.52–7.29), 2.97 for dienogest (95 % CI 0.96–9.24), 1.57 for drospirenone (95 % CI 0.46–5.38), 2.54 for chlormadinone (95 % CI 0.72–9.04), and 3.24 for norgestimate (95 % CI 0.59–17.75) compared to levonorgestrel. None of those findings reached statistical significance. The maximum absolute increase versus levonorgestrel was 6 cases per 10,000 women (n.s.).
Conclusion
The study shows the low incidence rates of thrombosis in OC users. Since there is no significant difference, this study does not confirm an increased risk but shows only a tendency for this risk of third- and fourth-generation OC versus levonorgestrel-containing products.



Similar content being viewed by others
References
Szarewski A, Mansour D (1999) The ‘pill scare’: the responses of authorities, doctors and patients using oral contraception. Hum Reprod Update 5(6):627–632
Khader YS, Rice J, John L, Abueita O (2003) Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception 68(1):11–17
World Health Organization (1995) Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case–control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 346(8990):1575–1582
Rabe T, Luxembourg B, Ludwig M et al (2011) Contraception and thrombophilia—a statement from the German Society of Gynecological Endocrinology and Reproductive Medicine (DGGEF e. V.) and the Professional Association of the German Gynaecologists (BVF e. V.). J Reprod Med Endocrinol 8(Sonderheft 1):178–218
Wu C, Grandi S, Filion K, Abenhaim H, Joseph L, Eisenberg M (2013) Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG 120(7):801–811
Plu-Bureau G, Maitrot-Mantelet L, Hugon-Rodin J, Canonico M (2013) Hormonal contraceptives and venous thromboembolism: an epidemiological update. Best Pract Res Clin Endocrinol Metab 27(1):25–34
Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E (2011) Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ 343:d6423
Dinger JC, Heinemann LA, Kühl-Habich D (2007) The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on Oral Contraceptives based on 142,475 women-years of observation. Contraception 75:344–354
Dinger J, Assmann A, Möhner S et al (2010) Risk of venous thromboembolism and the use of dienogest- and drospirenone-containing oral contraceptives: results from a German case–control study. J Fam Plann Reprod Health Care 36:123–129
Farmer RD, Todd JC, Lewis MA et al (1998) The risks of venous thromboembolic disease among German women using oral contraceptives: a database study. Contraception 57:67–70
Seeger JD, Loughlin J, Eng PM et al (2007) Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstet Gynecol 110:587–593
Jick H, Jick SS, Gurewich V et al (1995) Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestogen components. Lancet 346:1589–1593
van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJM, Rosendaal FR (2009) The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case–control study. BMJ 339:b2921
Lenzer J (2011) US panel rules that warnings on two birth control pills be strengthened. BMJ 343:d8104
Kostev K, Haas G (2011) Medical care in Germany. Optimus, Göttingen
Becher H, Kostev K, Schröder-Bernhardi D (2009) Validity and representativeness of the “Disease Analyzer” patient database for use in pharmaco-epidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther 47(10):617–626
Clayton TC, Gaskin M, Meade TW (2011) Recent respiratory infection and risk of venous thromboembolism: case–control study through a general practice database. Int J Epidemiol 40(3):819–827
Nightingale AL, Lawrenson RA, Simpson EL (2000) The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care 5(4):265–274
Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: does the type of progestogen make a difference? Contraception. 2000;62(2 Suppl):21S–28S (discussion 37S–38S)
Farmer RD, Lawrenson RA, Todd JC, Williams TJ, MacRae K (1999) Oral contraceptives and venous thromboembolic disease. Analyses of the UK General Practice Research Database and the UK Mediplus database. Hum Reprod Update 5(6):688–706
Motheral B, Brooks J, Clark MA (2003) A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value Health 6(2):90–97
Inman WHW, Vessey MP, Westerholm B, Engelund A (1970) Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. BMJ 2:203–209
Hannaford PC (2011) The progestogen content of combined oral contraceptives and venous thromboembolic risk. BMJ 25(343):d6592
Fazio G, Ferrara F, Barbaro G (2010) Prothrombotic effects of contraceptives. Curr Pharm Des 16(31):3490–3496
Thorneycroft IH (1999) Update on androgenicity. Am J Obstet Gynecol 180(2 Pt 2):288–294
Leblanc ES, Laws A (1999) Benefits and risks of third-generation oral contraceptives. J Gen Intern Med 14(10):625–632
Shapiro S, Dinger J (2010) Risk of VTE among users of oral contraceptives. J Fam Plann Reprod Health Care 36(2):103
Birkhäuser M, Braendle W, Kuhl H, Mück AO, Neulen J, Thaler C, Wildt L (2011) Aktuelle Empfehlungen zur hormonalen Kontrazeption—44. Arbeitstreffen des “Zürcher Gesprächskreises” vom Mai 2010. Journal für Gynäkologische Endokrinologie 5(1):17–25
Dinger J (2009) Oral contraceptives and venous thromboembolism: old questions revisited. J Fam Plann Reprod Health Care 35(4):211–213
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziller, M., Ziller, V., Haas, G. et al. Risk of venous thrombosis in users of hormonal contraceptives in German gynaecological practices: a patient database analysis. Arch Gynecol Obstet 289, 413–419 (2014). https://doi.org/10.1007/s00404-013-2983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2983-9