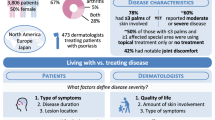
Abstract
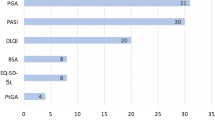
Psoriatic arthritis (PsA) is rarely assessed in psoriasis randomized controlled trials (RCT); thus, the effect of psoriasis therapy on PsA is unknown. The International Dermatology Outcome Measures (IDEOM) has included “PsA Symptoms” as part of the core domains to be measured in psoriasis RCT. This study aimed to achieve consensus about screening for PsA and how to measure for “PsA Symptoms” in psoriasis RCT. At the IDEOM 2017 Annual Meeting, stakeholders voted on the role of PsA screening in psoriasis RCT. To select measures for “PsA Symptoms”, we adapted the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) guidelines. Three potential measures were selected. At the meeting, stakeholders voted on the validity, feasibility, and responsiveness of these measures. Of the 47 stakeholders, 93% voted that all psoriasis trial participants should be screened for PsA. “PsA Symptoms” measures included Patient Global (PG)-arthritis, Routine Assessment Patient Index Data (RAPID)-3, and Psoriatic Arthritis Impact of Disease (PsAID)-9. During the voting, more than 50% of the voters agreed that RAPID3 and PsAID9 were good measures for PsA Symptoms, able to capture all its essential elements. PsAID9 was considered the most feasible instrument, followed by RAPID3 and PG-arthritis, respectively. Finally, most participants agreed that RAPID3 and PsAID9 were responsive measures. Most study participants voted that all subjects in a psoriasis clinical trial should be screened for PsA. RAPID3 and PsAID9 outperformed PG-arthritis in measuring PsA Symptoms. This will be followed by a Delphi survey involving a larger stakeholder group.





Similar content being viewed by others
References
Boehncke WH, Menter A (2013) Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol 14:377–388. https://doi.org/10.1007/s40257-013-0032-x
Callis Duffin K, Gottlieb AB, Merola JF, Latella J, Garg A, Armstrong AW (2017) Defining outcome measures for psoriasis: the IDEOM Report from the GRAPPA 2016 annual meeting. J Rheumatol 44:701–702. https://doi.org/10.3899/jrheum.170151
Cauli A, Gladman DD, Mathieu A, Olivieri I, Porru G, Tak PP, Sardu C, Ujfalussy I, Scarpa R, Marchesoni A (2011) Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol 38:898–903
Cauli A, Gladman DD, Mathieu A, Olivieri I, Porru G, Tak PP, Sardu C, Ujfalussy I, Scarpa R, Marchesoni A, Taylor WJ, Spadaro A, Fernandez-Sueiro JL, Salvarani C, Kalden JR, Lubrano E, Carneiro S, Desiati F, Flynn JA, D’Angelo S, Vacca A, AW VANK, Catanoso MG, Gruenke M, Peluso R, Parsons WJ, Ferrara N, Contu P, Helliwell PS, Mease PJ (2011) Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol 38:898–903. https://doi.org/10.3899/jrheum.100857
Coates L (2015) Outcome measures in psoriatic arthritis. Rheum Dis Clin N Am 41:699–710. https://doi.org/10.1016/j.rdc.2015.07.009
Coates L, Aslam T, Al Balushi F, Burden A, Burden-The E, Caperon A, Cerio R, Chattopadhyay C, Chinoy H, Goodfield M (2013) Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol 168:802–807
Coates L, Tillett W, Pincus T, Kavanaugh A, Helliwell P (2016) THU0419 Rapid3 performs well identifying treatment effect in the tight control of psoriatic arthritis study. BMJ Publishing Group Ltd, London
Coates LC, Tillett W, Pincus T, Kavanaugh A, Helliwell PS (2016) RAPID3 near remission shows good agreement with minimal disease activity criteria in psoriatic arthritis [abstract]. Arthritis Rheumatol 68(suppl 10)
Coates LC, Walsh J, Haroon M, FitzGerald O, Aslam T, Al Balushi F, Burden A, Burden-Teh E, Caperon AR, Cerio R (2014) Development and testing of new candidate psoriatic arthritis screening questionnaires combining optimal questions from existing tools. Arthritis Care Res 66:1410–1416
Coster WJ (2013) Making the best match: selecting outcome measures for clinical trials and outcome studies. Am J Occup Ther 67:162–170. https://doi.org/10.5014/ajot.2013.006015
de Wit M, Kvien T, Gossec L (2015) Patient participation as an integral part of patient-reported outcomes development ensures the representation of the patient voice: a case study from the field of rheumatology. RMD Open 1:e000129
de Wit MP, Kvien TK, Gossec L (2015) Patient participation as an integral part of patient-reported outcomes development ensures the representation of the patient voice: a case study from the field of rheumatology. RMD Open 1:e000129. https://doi.org/10.1136/rmdopen-2015-000129
Di Carlo M, Becciolini A, Lato V, Crotti C, Favalli EG, Salaffi F (2017) The 12-item Psoriatic Arthritis Impact of Disease Questionnaire: construct validity, reliability, and interpretability in a clinical setting. J Rheumatol 44:279–285. https://doi.org/10.3899/jrheum.160924
Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD (2015) Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res 67:264–272. https://doi.org/10.1002/acr.22401
Elman SA, Merola JF, Armstrong AW, Duffin KC, Latella J, Garg A, Gottlieb AB (2017) The International Dermatology Outcome Measures (IDEOM) Initiative: a review and update. J Drugs Dermatol 16:119–124
Favier G, Gladman DD, Merola JF, Armstrong AW, Boehncke WH, Helliwell PS (2017) Benchmarking care in psoriatic arthritis—the QUANTUM Report: a report from the GRAPPA 2016 annual meeting. J Rheumatol 44:674–678. https://doi.org/10.3899/jrheum.170142
Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R, Maccarone M, Carton L, Otsa K, Sooaar I, Heiberg T, Bertheussen H, Canete JD, Sanchez Lombarte A, Balanescu A, Dinte A, de Vlam K, Smolen JS, Stamm T, Niedermayer D, Bekes G, Veale D, Helliwell P, Parkinson A, Luger T, Kvien TK (2014) A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 73:1012–1019. https://doi.org/10.1136/annrheumdis-2014-205207
Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JY, Van Voorhees AS, Elmets CA, Leonardi CL, Beutner KR, Bhushan R, Menter A (2008) Guidelines of care for the management of psoriasis and psoriatic arthritis: Sect. 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 58:851–864. https://doi.org/10.1016/j.jaad.2008.02.040
Haroon M, Kirby B, FitzGerald O (2013) High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 72:736–740. https://doi.org/10.1136/annrheumdis-2012-201706
Helliwell P, Coates L, Chandran V, Gladman D, de Wit M, FitzGerald O, Kavanaugh A, Strand V, Mease PJ, Boehncke WH, Langley RG, Lubrano E, Maccarone M, Schulze-Koops H, Miceli-Richard C, Queiro R (2014) Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res 66:1759–1766. https://doi.org/10.1002/acr.22404
Hojgaard P, Klokker L, Orbai AM, Holmsted K, Bartels EM, Leung YY, Goel N, de Wit M, Gladman DD, Mease P, Dreyer L, Kristensen LE, FitzGerald O, Tillett W, Gossec L, Helliwell P, Strand V, Ogdie A, Terwee CB, Christensen R (2017) A systematic review of measurement properties of patient reported outcome measures in psoriatic arthritis: a GRAPPA-OMERACT initiative. Semin Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2017.09.002
Husni ME, Meyer KH, Cohen DS, Mody E, Qureshi AA (2007) The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol 57:581–587
Ibrahim G, Buch M, Lawson C, Waxman R, Helliwell P (2009) Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol 27:469
Leung YY, Ho KW, Zhu TY, Tam LS, Kun EW, Li EK (2012) Construct validity of the modified numeric rating scale of patient global assessment in psoriatic arthritis. J Rheumatol 39:844–848. https://doi.org/10.3899/jrheum.110919
Mease PJ (2011) Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res 63(Suppl 11):S64–S85. https://doi.org/10.1002/acr.20577
Michelsen B, Fiane R, Diamantopoulos AP, Soldal DM, Hansen IJ, Sokka T, Kavanaugh A, Haugeberg G (2015) A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PloS One 10:e0123582. https://doi.org/10.1371/journal.pone.0123582
Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, Terwee CB (2017) COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. https://doi.org/10.1007/s11136-017-1765-4
Mokkink LB, Prinsen CA, Bouter LM, Vet HC, Terwee CB (2016) The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz J Phys Ther 20:105–113. https://doi.org/10.1590/bjpt-rbf.2014.0143
Mokkink LB, Terwee CB, Knol DL, Stratford PW, Alonso J, Patrick DL, Bouter LM, de Vet HC (2010) The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol 10:22. https://doi.org/10.1186/1471-2288-10-22
Ogdie A, Weiss P (2015) The epidemiology of psoriatic arthritis. Rheum Dis Clin N Am 41:545–568. https://doi.org/10.1016/j.rdc.2015.07.001
Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, Tillett W, Elmamoun M, Callis Duffin K, Campbell W, Christensen R, Coates L, Dures E, Eder L, FitzGerald O, Gladman D, Goel N, Grieb SD, Hewlett S, Hoejgaard P, Kalyoncu U, Lindsay C, McHugh N, Shea B, Steinkoenig I, Strand V, Ogdie A (2017) International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis 76:673–680. https://doi.org/10.1136/annrheumdis-2016-210242
Orbai AM, Ogdie A (2016) Patient-reported outcomes in psoriatic arthritis. Rheum Dis Clin N Am 42:265–283. https://doi.org/10.1016/j.rdc.2016.01.002
Perez-Chada LM, Singh S, Callis-Duffin K, Garg A, Gottlieb AB, Latella J, Armstrong AW, Merola JF (2017) International Dermatology Outcome Measures (IDEOM) Group 2016 New York Meeting: meeting summary and data from the Psoriasis Working Group. J Drugs Dermatol 16:770–777
Porter ME, Larsson S, Lee TH (2016) Standardizing patient outcomes measurement. N Engl J Med 374:504–506. https://doi.org/10.1056/NEJMp1511701
Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, Williamson PR, Terwee CB (2016) How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”—a practical guideline. Trials 17:449
Salaffi F, Di Carlo M, Carotti M, Farah S, Gutierrez M (2016) The Psoriatic Arthritis Impact of Disease 12-item questionnaire: equivalence, reliability, validity, and feasibility of the touch-screen administration versus the paper-and-pencil version. Ther Clin Risk Manag 12:631–642. https://doi.org/10.2147/tcrm.s101619
Talli S, Etcheto A, Fautrel B, Balanescu A, Braun J, Canete JD, de Vlam K, de Wit M, Heiberg T, Helliwell P, Kalyoncu U, Kiltz U, Maccarone M, Niedermayer D, Otsa K, Scrivo R, Smolen JS, Stamm T, Veale DJ, Kvien TK, Gossec L (2016) Patient global assessment in psoriatic arthritis - what does it mean? An analysis of 223 patients from the Psoriatic arthritis impact of disease (PsAID) study. Joint Bone Spine 83:335–340. https://doi.org/10.1016/j.jbspin.2015.06.018
Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC (2012) Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 21:651–657. https://doi.org/10.1007/s11136-011-9960-1
Tom BD, Chandran V, Farewell VT, Rosen CF, Gladman DD (2015) Validation of the Toronto psoriatic arthritis screen version 2 (ToPAS 2). J Rheumatol 42:841–846
Vakil-Gilani K, Dinno A, Garg N, Deodhar AA (2015) Routine Assessment of Patient Index Data 3 Score (rapid3) and Psoriasis Quality of Life (pqol-12) Assess Different Domains in Psoriasis (pso) and Psoriatic Arthritis (psa) Patients. Arthritis Rheumatol 67:897–898
Yazici Y, Bergman M, Pincus T (2008) Time to score quantitative rheumatoid arthritis measures: 28-Joint Count, Disease Activity Score, Health Assessment Questionnaire (HAQ), Multidimensional HAQ (MDHAQ), and Routine Assessment of Patient Index Data (RAPID) scores. J Rheumatol 35:603–609
Acknowledgements
We thank Dr. Philip Mease for his collaboration in the design of the Delphi Survey during the 2018 GRAPPA adjacent to the AAD Meeting in San Diego, CA, USA.
Funding
This study was funded by the International Dermatology Outcome Measures group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gottlieb is an advisor/consultant for Janssen Inc.; Celgene Corp., Bristol Myers Squibb Co., Beiersdorf, Inc., Abbvie, UCB, Novartis, Incyte, Pfizer, Lilly, Xenoport, Development Crescendo Bioscience, Aclaris, Amicus, Reddy Labs, Valeant, Dermira, Allergan, CSL Behring, Merck, Sun Pharmaceutical Industries. Also, she received research/educational grants from Janssen, Incyte. Dr. Duffin has been a consultant and/or investigator for Amgen, Janssen, Lilly, Novartis, Celgene, Pfizer; Bristol-Myers Squibb Co. Dr. Garg served on the advisory board of Abbvie, Janssen, and Pfizer receiving honoraria. Also, he received research/educational grants from AbbVie and Merck. Dr. Armstrong has served as investigator, advisor and/or consultant to AbbVie, Janssen, Novartis, Lilly, Regeneron, Sanofi, Science 37, Modernizing Medicine, and Valeant. Dr. Merola has served as an advisor/consultant for Biogen IDEC, AbbVie, Amgen, Eli Lilly, Novartis, Pfizer, Janssen, UCB, Kiniksa, Momenta and Mallinckrodt. He has been a speaker for AbbVie and Eli Lilly, and an investigator for Biogen IDEC, Amgen, Pfizer and Boehringer Ingelheim. Dr. Ogdie served as a consultant for Bristol-Myers Squibb, Lilly, Novartis, Pfizer, and Takeda and has received grant funding to the University of Pennsylvania from Pfizer (co-investigator) and Novartis. John Latella has served as Patient Consultant for Boehringer Ingelheim, and Patient Advocate for GfK. Dr. Perez-Chada has received research funding from “RADLA Scholarship 2010”. The other authors have no disclosures.
Research involving human participants and/or animals
Evaluation by an ethical board was deemed exempt given the nature of a consensus meeting. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Process to obtain informed consent was deemed exempt given the nature of a consensus meeting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
403_2018_1855_MOESM3_ESM.pdf
Online Resource 3: Pubmed search strategy to identify articles reporting the psychometric properties of the PG-arthritis VAS, PG-arthritis NRS, RAPID3, PsAID9 and PsAID12 (PDF 99 KB)
403_2018_1855_MOESM5_ESM.pdf
Overview and summary of the psychometric properties of the PG-arthritis VAS, PG-arthritis NRS, RAPID3, PsAID9 and PsAID12 (PDF 242 KB)
Rights and permissions
About this article
Cite this article
Perez-Chada, L.M., Cohen, J.M., Gottlieb, A.B. et al. Achieving international consensus on the assessment of psoriatic arthritis in psoriasis clinical trials: an International Dermatology Outcome Measures (IDEOM) initiative. Arch Dermatol Res 310, 701–710 (2018). https://doi.org/10.1007/s00403-018-1855-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-018-1855-3