Abstract
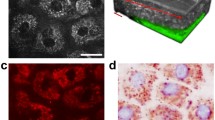
Ehlers–Danlos syndrome (EDS) is the name for a heterogenous group of rare genetic connective tissue disorders with an overall incidence of 1 in 5000. The histological characteristics of EDS have been previously described in detail in the late 1970s and early 1980s. Since that time, the classification of EDS has undergone significant changes, yet the description of the histological features of collagen morphology in different EDS subtypes has endured the test of time. Nonlinear microscopy techniques can be utilized for non-invasive in vivo label-free imaging of the skin. Among these techniques, two-photon absorption fluorescence (TPF) microscopy can visualize endogenous fluorophores, such as elastin, while the morphology of collagen fibers can be assessed by second-harmonic generation (SHG) microscopy. In our present work, we performed TPF and SHG microscopy imaging on ex vivo skin samples of one patient with classical EDS and two patients with vascular EDS and two healthy controls. We detected irregular, loosely dispersed collagen fibers in a non-parallel arrangement in the dermis of the EDS patients, while as expected, there was no noticeable impairment in the elastin content. Based on further studies on a larger number of patients, in vivo nonlinear microscopic imaging could be utilized for the assessment of the skin status of EDS patients in the future.





Similar content being viewed by others
References
Beighton P, de Paepe A, Danks D, Finidori G, Gedde-Dahl T, Goodman R, Hall JG, Hollister DW, Horton W, McKusick VA et al (1988) International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet 29:581–594. https://doi.org/10.1002/ajmg.1320290316
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 77:31–37
Carlesimo M, Cortesi G, Gamba A, Narcisi A, Turturro F, Raffa S, Torrisi MR, Camplone G (2012) Ehlers-Danlos syndrome: case report and an electron microscopy study. Rheumat Int 32:1507–1510. https://doi.org/10.1007/s00296-010-1778-6
Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A (2017) A framework for the classification of joint hypermobility and related conditions. Am J Med Genet Part C Semin Med Genet 175:148–157. https://doi.org/10.1002/ajmg.c.31539
Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ (2012) Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7:654–669. https://doi.org/10.1038/nprot.2012.009
Cicchi R (2014) The new digital pathology: just say NLO. Dig Dis Sci 59:1347–1348. https://doi.org/10.1007/s10620-014-3165-8
Cicchi R, Kapsokalyvas D, Pavone FS (2014) Clinical nonlinear laser imaging of human skin: a review. Biomed Res Int 2014:903589. https://doi.org/10.1155/2014/903589
Cui JZ, Tehrani AY, Jett KA, Bernatchez P, van Breemen C, Esfandiarei M (2014) Quantification of aortic and cutaneous elastin and collagen morphology in Marfan syndrome by multiphoton microscopy. J Struct Biol 187:242–253. https://doi.org/10.1016/j.jsb.2014.07.003
Giunta C, Nuytinck L, Raghunath M, Hausser I, De Paepe A, Steinmann B (2002) Homozygous Gly530Ser substitution in COL5A1 causes mild classical Ehlers-Danlos syndrome. Am J Med Genet 109:284–290. https://doi.org/10.1002/ajmg.10373
Giunta C, Steinmann B (2000) Compound heterozygosity for a disease-causing G1489E [corrected] and disease-modifying G530S substitution in COL5A1 of a patient with the classical type of Ehlers-Danlos syndrome: an explanation of intrafamilial variability? Am J Med Genet 90:72–79
Haluszka D, Lorincz K, Kiss N, Szipocs R, Kuroli E, Gyongyosi N, Wikonkal NM (2016) Diet-induced obesity skin changes monitored by in vivo SHG and ex vivo CARS microscopy. Biomed Opt Express 7:4480–4489. https://doi.org/10.1364/BOE.7.004480
Haluszka D, Lorincz K, Molnar G, Tamas G, Kolonics A, Szipocs R, Karpati S, Wikonkal NM (2015) In vivo second-harmonic generation and ex vivo coherent anti-stokes raman scattering microscopy to study the effect of obesity to fibroblast cell function using an Yb-fiber laser-based CARS extension unit. Microsc Res Tech 78:823–830. https://doi.org/10.1002/jemt.22545
Hermanns-Lê T, Reginster M-A, Piérard-Franchimont C, Piérard GE (2012) Ehlers–Danlos syndrome. In: Stirling JW, Curry A, Eyden B (eds) Diagnostic electron microscopy—a practical guide to interpretation and technique. Wiley, pp 309–321. https://doi.org/10.1002/9781118452813.ch12
Iurassich S, Rocco D, Aurilia A (2001) Type III Ehlers-Danlos syndrome: correlations among clinical signs, ultrasound, and histologic findings in a study of 35 cases. Int J Dermatol 40:175–178
Lacomb R, Nadiarnykh O, Campagnola PJ (2008) Quantitative second harmonic generation imaging of the diseased state osteogenesis imperfecta: experiment and simulation. Biophys J 94:4504–4514. https://doi.org/10.1529/biophysj.107.114405
Lorincz K, Haluszka D, Kiss N, Gyongyosi N, Banvolgyi A, Szipocs R, Wikonkal NM (2017) Voluntary exercise improves murine dermal connective tissue status in high-fat diet-induced obesity. Arch Dermatol Res 309:209–215. https://doi.org/10.1007/s00403-017-1715-6
Malfait F, Coucke P, Symoens S, Loeys B, Nuytinck L, De Paepe A (2005) The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat 25:28–37. https://doi.org/10.1002/humu.20107
Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP, Castori M, Cohen H, Colombi M, Demirdas S, De Backer J, De Paepe A, Fournel-Gigleux S, Frank M, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B (2017) The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet Part C Semin Med Genet 175:8–26. https://doi.org/10.1002/ajmg.c.31552
Mansfield J, Yu J, Attenburrow D, Moger J, Tirlapur U, Urban J, Cui Z, Winlove P (2009) The elastin network: its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J Anat 215:682–691. https://doi.org/10.1111/j.1469-7580.2009.01149.x
Mayer K, Kennerknecht I, Steinmann B (2013) Clinical utility gene card for: Ehlers–Danlos syndrome types I–VII and variants—update 2012. Eur J Hum Genet 21. https://doi.org/10.1038/ejhg.2012.162
Mitchell AL, Schwarze U, Jennings JF, Byers PH (2009) Molecular mechanisms of classical Ehlers-Danlos syndrome (EDS). Hum Mutat 30:995–1002. https://doi.org/10.1002/humu.21000
Murata T, Honda T, Miyachi Y, Kabashima K (2013) Morphological character of pseudoxanthoma elasticum observed by multiphoton microscopy. J Dermatol Sci 72:199–201. https://doi.org/10.1016/j.jdermsci.2013.06.016
Parapia LA, Jackson C (2008) Ehlers-Danlos syndrome—a historical review. Br J Haematol 141:32–35. https://doi.org/10.1111/j.1365-2141.2008.06994.x
Pierard GE, Pierard-Franchimont C, Lapiere CM (1983) Histopathological aid at the diagnosis of the Ehlers-Danlos syndrome, gravis and mitis types. Int J Dermatol 22:300–304
Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA (1975) Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci USA 72:1314–1316
Ranjit S, Dvornikov A, Stakic M, Hong SH, Levi M, Evans RM, Gratton E (2015) Imaging fibrosis and separating collagens using second harmonic generation and phasor approach to fluorescence lifetime imaging. Sci Rep 5. https://doi.org/10.1038/srep13378
Ricard-Blum S (2011) The Collagen family. Cold Spring Harb Perspect Biol 3. https://doi.org/10.1101/cshperspect.a004978
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78:929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Sulica VI, Cooper PH, Pope FM, Hambrick GW Jr, Gerson BM, McKusick VA (1979) Cutaneous histologic features in Ehlers-Danlos syndrome: study of 21 patients. Arch Dermatol 115:40–42
Symoens S, Syx D, Malfait F, Callewaert B, De Backer J, Vanakker O, Coucke P, De Paepe A (2012) Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat 33:1485–1493. https://doi.org/10.1002/humu.22137
Tinkle B, Castori M, Berglund B, Cohen H, Grahame R, Kazkaz H, Levy H (2017) Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet Part C Semin Med Genet 175:48–69. https://doi.org/10.1002/ajmg.c.31538
Tzeng SY, Kuo TY, Hu SB, Chen YW, Lin YL, Chu KY, Tseng SH (2018) Skin collagen can be accurately quantified through noninvasive optical method: validation on a swine study. Skin Res Technol 24:59–64. https://doi.org/10.1111/srt.12390
Wang Y, Xu R, He W, Yao Z, Li H, Zhou J, Tan J, Yang S, Zhan R, Luo G, Wu J (2015) Three-Dimensional histological structures of the human dermis. Tissue Eng Part C Methods 21:932–944. https://doi.org/10.1089/ten.TEC.2014.0578
Yang JH, Lee ST, Kim JA, Kim SH, Jang SY, Ki CS, Kim DK (2007) Genetic analysis of three Korean patients with clinical features of Ehlers-Danlos syndrome type IV. J Korean Med Sci 22:698–705. https://doi.org/10.3346/jkms.2007.22.4.698
Acknowledgements
Above all, we would like to thank the patients and the control subjects for their cooperation and for providing skin samples and molecular genetic data for this study. We acknowledge the support of Fanni Virág Ralovich and Krisztina Horváth in the collection of the clinical data and Mercédesz Mazán and Flóriánné Adrien Suba for their assistance in the genetic screening.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kiss, N., Haluszka, D., Lőrincz, K. et al. Ex vivo nonlinear microscopy imaging of Ehlers–Danlos syndrome-affected skin. Arch Dermatol Res 310, 463–473 (2018). https://doi.org/10.1007/s00403-018-1835-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-018-1835-7