Abstract
Background
UKA necessitates a learning period. From this point of view, it would be logical to prefer the design that tolerates suboptimal tibial rotations better, especially for inexperienced surgeons. The aim of this study was to evaluate and compare the clinical and radiological results of mobile-bearing and fix-bearing UKA designs in case of suboptimal tibial rotations.
Methods
A retrospective case–control evaluation was made of all the patients with medial compartment osteoarthritis, treated between January 2011 and January 2015. 324 patients ideal femoral rotation were enrolled in the study. 153 patients (Group 1) were treated with fix-bearing design with a mean 28.8 ± 11.3 month follow-up and 171 patients (Group 2) were treated with mobile-bearing design with a 31 ± 14.3 month follow-up. Each patient in groups was subdivided into (A): optimal tibial rotation, (B): external rotation of tibial component > 5°, (C): internal rotation of tibial component > 5° subgroups. WOMAC and KSS scores of each patient at preoperative and postoperative final control were compared between groups and subgroups.
Results
No significant differences were determined between the groups in terms of mean follow-up time (p = 0.0612), preoperative WOMAC, and KSS scores (p = 0.754 and p = 0.832, respectively). No significant differences were determined between subgroups 1A and 2A in terms of WOMAC and KSS scores at the final evaluation (p = 0.314 and p = 0.546, respectively). A significant difference was determined between subgroups 1B and 2B in terms of WOMAC and KSS scores (p = 0.021 and p = 0.012, respectively). In addition, the difference between subgroups 1C and 2C was significant (p = 0.047 and p = 0.034, respectively) at the final evaluation.
Conclusion
Both mobile- and fix-bearing designs are beneficial in the treatment of medial compartment osteoarthritis of the knee. However, in case of both tibial internal or external suboptimal tibial rotations, fix-bearing design have better results compared to mobile-bearing design.
Study design
Level III retrospective comparative clinical study.
Similar content being viewed by others
Introduction
Unicompartmental knee arthroplasty (UKA) is an effective treatment for single-compartment osteoarthritis [1,2,3]. On the other hand, proper surgical technique, optimal implant replacement, and positioning are essential to obtain a satisfactory outcome [4, 5]. If technical steps are ignored, catastrophic result could occur. Especially ensuring the right rotation of the implants is essential for long-term survival and an inaccurate implantation is considered a factor for the early failure [6, 7].
In addition, UKA necessitates a learning period for all orthopaedic surgeons [8]. At beginning of the period, the surgeon is considered to be at the baseline level of surgical skill and at the end the surgeon is in an expertise level. The learning process varies depending on surgeon-related factors (speed of learning, previous experiences, and surgical skill), institutional factors (operating team experience, the size of the institution, and caseload volume), and financial resources [9, 10].
There are two basic designs in UKA: fixed and bearing, and each design has its own advantages and disadvantages [11, 12]. From this point of view, it would be logical for inexperienced surgeons, to prefer the design that better tolerates suboptimal tibial rotations. The aim of this study was to evaluate and compare the clinical and radiological results of mobile- and fix-bearing UKA designs in case of suboptimal tibial rotations.
Patients and methods
A retrospective case–control evaluation was made of all the patients with medial compartment osteoarthritis who were treated between January 2011 and January 2015.
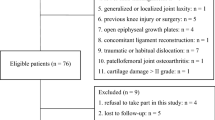
The indications for inclusion in this study were that patients had undergone primary elective unilateral UKA with fixed bearing (ZIMMER®, Warsaw, Indiana, USA) and mobile bearing (BIOMET®, Warsaw, Indiana, USA) with at least 2 years of follow-up, safe mobilization with or without support, were able to meet their own personal needs, and had no wound site problems and no major complications or inflammatory disease. Patients included in the study all had varus knee and medial compartmental arthritis. Patients were excluded from the study if there were missing records or if there was no voluntary informed consent form.
360 patients met the criteria and were called for a final evaluation; CT of knee for each patient was obtained, and femoral and tibial rotations of the components were measured. 324 patients with ideal femoral rotation were enrolled in the study. 153 patients (Group 1) were treated with fix-bearing design with a mean 28.8 ± 11.3 month follow-up and 171 patients (Group 2) were treated with mobile-bearing design with a 31 ± 14.3 month follow-up. Both groups were subdivided into (A): optimal tibial rotation (between 0° and 5° internal or external rotation), (B): external rotation of tibial component > 5°, and (C): internal rotation of tibial component > 5° subgroups. The KSS, KSS-F, WOMAC-P scores, and ROM measurements of each patient at preoperative and postoperative final controls were compared between groups and subgroups.
Our routine clinical practice content includes preoperative examination (range of motion, KSS, and WOMAC scoring) and follow-up examinations. All measurements were made by the same physiotherapist group and data were recorded in the patient registry. The KSS, KSS-F, WOMAC-P scores, and ROM measurements were calculated for each patient. Each variable values at preoperative and postoperative final control, were compared between groups and subgroups.
All patients were operated on by the same surgical team. There was no differential indication on choosing whether fixed or mobile-bearing UKA and the concept changed for all consecutive patients. In all knees, the femoral components and the tibial components were cemented. The same surgical technique was applied to all the patients. The first bone cut was made from the tibia. Patelloplasty and patellar denervation was applied to all patients; no patient required a patellar implant. The proximal tibial bone cut was performed first. The tibial cut should be perpendicular to the tibial mechanical axis. In the rotation of the tibial component, the Akagi line, tibial crest, and midpoint of the ankle were used as reference [13, 14]. Placement of the tibial component was achieved with as much cortical fit as possible in the antero-posterior and medio-lateral planes. In addition, for tibial sizing and setting, the tibial template was always placed against the osteotomized tibial plane to visually determine the maximum coverage with the osteotomized tibial plateau. The trial prosthesis was inserted to check rotation. Priority was always given to correction of the tibial rotational positioning and maximum tibial coverage. An entry hole was drilled 1 cm superior–inferior to the PCL. Femoral cutting block was placed using intramedullary rod and distal femoral cut was made. The chamfer and posterior femoral cuts were made using guide with the knee flexed to 90°.
The drains in the patients were removed after 24 h. Then, by starting strenuous knee ROM exercises, and active and passive knee exercises in the bed, mobilization was provided. On postoperative day 1, it was aimed to reach 120° knee flexion and self-mobilization without support.
From the computed tomography (CT) images taken at the final follow-up examination, digital measurements were taken and recorded in millimeters to evaluate the rotation of tibial components. In the radiological evaluation of the patients at the final follow-up examination, CT images were taken at 0.6 mm slice thickness with metal artifact eliminating software (256 slices multidetector scanner; Siemens®, Erlangen, Germany). Each CT image of the patients was examined by radiologists experienced in musculoskeletal system radiology with Leonardo Dr/Dsa Va30a software (Siemens®, Erlangen, Germany). To reduce inter-observer and intra-observer errors, the images of the patients were evaluated double-blind by three different orthopaedic surgeons. The maximum errors of the measurements were determined as 0.7 mm and 1.2 using the current measurement techniques. Measurements were made to a sensitivity of 1/10 mm in the axial plane, of the tibial component rotational alignment (Fig. 1). The highest tolerance between all the measurements was determined as 0.7 mm. By calculating the tibial component rotation, standardization was obtained for males and females. Components placed in internal rotation > 5° to the medial third of the tibial tubercle were accepted as in internal rotation and components placed in external rotation > 5° to the medial third of the tibial tubercle were accepted as in external rotation (Fig. 2).
Conformity to normal distribution of the variables in the study to normal distribution was evaluated with the Shapiro–Wilk test. Descriptive statistics were used, and variables showing normal distribution were stated as mean ± standard deviation (SD) and those not showing normal distribution were stated as median (minimum–maximum). As additional information, mean ± SD were stated. Categorical variables such as gender, overhang status, and pain were shown as number (n) and percentage (%). The independent sample t test was used to examine the difference in age, BMI, and postoperative month values according to gender. In the comparison of the pre and postoperative values of KSS, KSS-F, WOMAC, and ROM, the Wilcoxon signed-rank test was used. The Pearson Chi-square test was applied to show any differences between the groups in respect of categorical variables. When the number of subjects was insufficient, number and percentage were stated. To examine the differences in the groups in respect of overhang, pain, and rotation, and the KSS, KSS-F, and WOMAC-P values, a method was used compatible with the Mann–Whitney U test and the Kruskal–Wallis non-parametric variance analysis. For variable values with a significant difference determined as a result of Kruskal–Wallis analysis, the analysis results were given after Bonferroni correction in the paired comparison of the groups. Alpha value of p ≤ 0.05 was considered significant.
Results
No significant differences were determined between the groups in terms of age and body mass index (BMI) (Table 1). No significant differences were determined between Group 1 and 2 in terms of mean follow-up time (p = 0.0612), preoperative WOMAC, and KSS scores (p = 0.754 and p = 0.832, respectively) (Table 2). In addition, no significant differences were determined between subgroups 1A and 2A in terms of WOMAC and KSS scores at postoperative final evaluation (p = 0.314 and p = 0.546, respectively). A significant difference was determined in case of suboptimal tibial rotation: the difference was significant between subgroups 1B and 2B in terms of WOMAC and KSS scores (p = 0.021 and p = 0.012, respectively). In addition, the difference between subgroups 1C and 2C was significant (p = 0.047 and p = 0.034, respectively) at the final evaluation (Table 3).
Discussion
The current study evaluated and compared the clinical and radiological results of mobile-bearing and fix-bearing UKA designs in case of suboptimal tibial rotations. It was found that better clinical results were obtained with fixed-bearing designs in case of suboptimal tibial rotations.
During implantation of both of femoral and tibial components, depending on the level of the tibia cut, the rotational compatibility of the components and correct sizing are crucial for a well performed, uncomplicated UKA [8, 15, 16]. Although the consequences of suboptimal tibial rotation in UKA have previously been analyzed, the literature seems to be extremely weak in respect of the effect on clinical outcome scores of such mistake. Traditionally, the placement of the tibial component in UKA has focused on maximizing coverage of the tibial surface. In the current series, it was aimed to provide maximum coverage and ideal rotation on tibia plateau. Implant under sizing could theoretically be harmful by leaving an uncovered cancellous bone surface [5]. Even if a tibial component is ideal in the medio-lateral dimension, it may be oversized in the antero-posterior dimension because of the limited design of tibial components. For optimal sizing, tibial components should be available in many sizes in both AP and ML dimensions. This point warrants further investigation, but may have possible implications for the design of these knee implants.
In case of the marked variation in external rotation position, our finding regarding tibial component rotation is consistent with the previous reports. Iriberri and Aragon reported that the tibial component exhibited an average angle of 11.9° of external rotation (− 1 to 32) [17]. They stated that sagittal tibial resection, which defines tibial component rotation, was performed using a free-hand technique with no anatomic landmark. A tibial component positioned in a neutral or slight external rotation showed better clinical outcomes than that with excessive external rotation. Campbell et al. reported that the tibial component was implanted with external rotations of 6.59° ± 7.23° and 5.68° ± 6.77° relative to the posterior cortical rim of the tibia and projected femoral transepicondylar axis, respectively [18]. As with the former study, Campbell et al. did not provide details of the sagittal tibial resection method. They reported that the greater variability in tibial component rotation was expected, as rotational alignment during the operation was essentially not instrumented. Although Kawahara et al. suggested that medial sixth of the patellar tendon at the tibial attachment is appropriate landmark for the anterior landmark of the tibial component rotation, rotating saw blade during sagittal tibial resection may produce variable rotational alignment [19]. Servien et al. reported that the mean tibial component rotation in 19 knees was 6.5° ± 5.1° of external rotation (range − 6.0 to 13.2) [20]. The sagittal tibial resection in their study was performed just lateral to the medial tibial plateau in the axial direction of the medial side of the notch. In other words, they performed the sagittal tibial resection along the medial wall of the intercondylar notch, as recommended by another study of Kawahara et al. [19].
Shakespeare et al. reported a tendency to greater internal rotation of the tibial component than the conventional method; moreover, the mean femoral rotation relative to the tibial component was reduced in terms not only of amplitude but also variability [21]. However, they measured the tibial component rotation indirectly via the femoral component shape in fully extended knees using simple radiographs. In a recent MRI study involving 90° knees, the lateral wall of the MFC was suggested as a landmark for sagittal tibial resection [22].
In the current study, the bearing rotational position relative to the lateral wall of the tibial component showed greater variability than the tibial component rotation itself. Ideally, the PE bearing should be located 1–2 mm medial to the lateral wall of the tibial component and coincident with the lateral wall [20, 21]. As reported by Shakespeare et al., divergent or convergent axial position between the long axes of the bearing and tibial component may result in impingement of the bearing against the lateral wall or free rotation of the bearing [21].
There have been a few studies that have studied the relationship of UKA axial rotational tibial component alignment [22]. This is, in part, due to the need for CT scans to measure axial rotation, which impart additional costs and radiation risks to patients. It has been suggested that tibial components should have neutral rotation in extension and slight internal rotation in flexion [23]. In the same 2D study, better outcomes were observed with lesser degrees of tibial external rotation, as excessive external rotation would result in a rotational incongruity between femur and tibia in extension [24]. Interestingly, an in vitro biomechanical study demonstrated significantly greater anterior/antero-medial strain in the tibia when tibial components were externally rotated by 10° than tibial components at neutral rotation of 0°, which, may in part, explain the lower functional outcomes in patients with higher degrees of external rotation [25].
Limited number of patients and retrospective design were the major limitations of the study. Another limitation was the absence of subgroup analyses, such as between-gender differences in age and BMI. As the study only included patients with knee arthritis based on a varus knee, the effect of suboptimal tibial rotation in patients in valgus knees was not evaluated. There were insufficient data on the subject of whether reverse asymmetrical tibial plateau morphometry or symmetrical tibial plateau morphometry caused suboptimal tibial placement as preoperative CT images had not been taken. Genetic and morphometric differences were not shown in the tibial tubercle, tibial crest, PCL tibial attachment site, tibial-ankle rotation, and epicondylar axis, which are used in the planning of rotational alignment. There are several limitations in the current study. Because the sample size was relatively small, correlation or multiple variable analyses could not be performed. Thus, the current findings should be confirmed in a larger cohort. Second, due to the lack of clinical outcomes, future well-designed studies that compare results according to tibial component rotation after medial UKA are required to assess the clinical relevance. Third, there is no scientific evidence regarding the relationship between bearing spinning and its dislocation. Finally, we did not determine the optimal tibial component rotation; therefore, this should be assessed in a future biomechanical study. Second, PE bearing movement does not always move along the lateral wall of the tibial component in parallel fashion. As shown by the instant bearing position, it seems to be imperative to inform other orthopaedic surgeons of possibility of PE bearing spinning in the mobile-bearing UKA. As a consequence, spun PE bearing may have a chance of dislocation or impingement to the tibial component lateral wall. Finally, iatrogenic injury to the PCL should be concerned during tibial bone resection. Although actual injury of the PCL should be studied clinically and biomechanically, sawing near the PCL tibial attachment is not infrequent according to our data.
To our knowledge, the current study is the first to demonstrate bearing spinning and PCL fossa involvement together with tibial component rotation in mobile-bearing medial UKA. With the current findings, further research into the relationship between tibial component rotation and PE bearing spinning is warranted. In three points, the clinical relevance of the current study can be suggested. Since it is too difficult to recognize the ASIS and maintain it as a guidance during the operation, determination of the tibial component rotation according to the ASIS should be prohibited. Although the “ideal” landmark for the tibial component rotation during the medial UKA has not yet been revealed, the structure of the proximal tibia, such as medial slope of the medial tibial spine or the lateral wall of the MFC, can be considered as an alternative landmark.
Both mobile-bearing and fix-bearing designs are beneficial in treatment of medial compartment osteoarthritis of the knee. However, in case of both tibial internal or external suboptimal tibial rotations, fix-bearing design has better results compared to mobile-bearing design. Thus, it could be thought that fix-bearing designs better tolerates suboptimal tibial rotations.
References
Kort NP, van Raay JJ, van Horn JJ (2007) The Oxford phase III unicompartmental knee replacement in patients less than 60 years of age. Knee Surg Sports Traumatol Arthrosc 15:356–360
Scott CEH, Wade FA, MacDonald D, Nutton RW (2018) Ten-year survival and patient-reported outcomes of a medial unicompartmental knee arthroplasty incorporating an all-polyethylene tibial component. Arch Orthop Trauma Surg 138:719–729
Tang H, Zhao L, Yan H, Jin D, Su X (2012) Mid-term effectiveness of Oxford unicompartmental knee system phase III for medial unicompartmental knee osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26:17–20
Kaya Bicer E, Servien E, Lustig S, Demey G, Ait Si Selmi T, Neyret P (2010) Sagittal flexion angle of the femoral component in unicompartmental knee arthroplasty: is it same for both medial and lateral UKAs? Knee Surg Sports Traumatol Arthrosc 18:928–933
Kim JG, Kasat NS, Bae JH, Kim SJ, Oh SM, Lim HC (2012) The radiological parameters correlated with the alignment of the femoral component after Oxford phase 3 unicompartmental knee replacement. J Bone Jt Surg Br 94:1499–1505
Bert JM (1998) 10-year survivorship of metal-backed, unicompartmental arthroplasty. J Arthroplast 13:901–905
Cartier P, Sanouiller JL, Grelsamer RP (1996) Unicompartmental knee arthroplasty surgery. 10-year minimum follow-up period. J Arthroplast 11:782e788
Zhang Q, Zhang Q, Guo W, Liu Z, Cheng L, Yue D, Zhang N (2014) The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LC-CUSUM). J Orthop Surg Res 6(9):81
Dionigi G, Bacuzzi A, Boni L, Rovera F, Dionigi R (2008) What is the learning curve for intraoperative neuromonitoring in thyroid surgery? Int J Surg 6:7–12
Walton R, Theodorides A, Molloy A, Melling D (2012) Is there a learning curve in foot and ankle surgery? Foot Ankle Surg 18:62–65
Peersman G, Stuyts B, Vandenlangenbergh T, Cartier P, Fennema P (2015) Fixed- versus mobile-bearing UKA: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 23:3296–3305
Peersman G, Slane J, Vuylsteke P, Fuchs-Winkelmann S, Dworschak P, Heyse T, Scheys L (2017) Kinematics of mobile-bearing unicompartmental knee arthroplasty compared to native: results from an in vitro study. Arch Orthop Trauma Surg 137:1557–1563
Akagi M, Matsusue Y, Mata T, Asada Y, Horiguchi M, Iida H, Nakamura T (1999) Effect of rotational alignment on patellar tracking in total knee arthroplasty. Clin Orthop Relat Res 366:155–163
Akagi M, Mori S, Nishimura S, Nishimura A, Asano T, Hamanishi C (2005) Variability of extraarticular tibial rotation references for total knee arthroplasty. Clin Orthop Relat Res 436:172–176
Kang KT, Son J, Baek C, Kwon OR, Koh YG (2018) Femoral component alignment in unicompartmental knee arthroplasty leads to biomechanical change in contact stress and collateral ligament force in knee joint. Arch Orthop Trauma Surg 138:563–572
Matziolis G, Mueller T, Layher F, Wagner A (2018) The femoral component alignment resulting from spacer block technique is not worse than after intramedullary guided technique in medial unicompartimental knee arthroplasty. Arch Orthop Trauma Surg 138:865–870
Iriberri I, Aragon JF (2014) Alignment of the tibial component of the unicompartmental knee arthroplasty, assessed in the axial view by CT scan: does it in uence the outcome? Knee 21:1269–1274
Campbell DG, Johnson LJ, West SC (2006) Multiparameter quantitative computer-assisted tomography assessment of uni-compartmental knee arthroplasties. ANZ J Surg 76:782–787
Kawahara S, Okazaki K, Matsuda S, Mitsuyasu H, Nakahara H, Okamoto S, Iwamoto Y (2014) Medial sixth of the patellar tendon at the tibial attachment is useful for the anterior reference in rotational alignment of the tibial component. Knee Surg Sports Traumatol Arthrosc 22:1070–1075
Servien E, Fary C, Lustig S, Demey G, Saffarini M, Chomel S, Neyret P (2011) Tibial component rotation assessment using CT scan in medial and lateral unicompartmental knee arthroplasty. Orthop Traumatol Surg Res 97:272–275
Shakespeare D, Ledger M, Kinzel V (2005) The influence of the tibial sagittal cut on component position in the Oxford knee. Knee 12:169–176
Goodfellow J (2006) Unicompartmental arthroplasty with the Oxford knee. Oxford University Press, Oxford
Gulati A, Pandit H, Jenkins C, Chao R, Dodd CA, Murray DW (2009) The effect of leg alignment on the outcome ofunicompartmental knee replacement. J Bone Jt Surg Br 91:469
Bell SW, Young P, Drury C, Smith J, Anthony I, Jones B, Blyth M, McLean A (2014) Component rotational alignment in unexplainedpainful primary total knee arthroplasty Knee 21:272
Small SR, Berend ME, Rogge RD, Archer DB, Kingman AL, Ritter MA (2013) Tibial loading after UKA: evaluation oftibial slope, resection depth, medial shift and component rotation. J Arthroplast 28:179
Funding
There is no funding source for the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ozcan, C., Simsek, M.E., Tahta, M. et al. Fixed-bearing unicompartmental knee arthroplasty tolerates higher variance in tibial implant rotation than mobile-bearing designs. Arch Orthop Trauma Surg 138, 1463–1469 (2018). https://doi.org/10.1007/s00402-018-3005-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-3005-y