Abstract
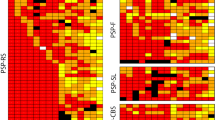
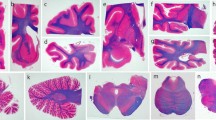
Corticobasal degeneration (CBD) is a clinically heterogeneous tauopathy, which has overlapping clinicopathologic and genetic characteristics with progressive supranuclear palsy (PSP). This study aimed to elucidate whether transactive response DNA-binding protein of 43 kDa (TDP-43) pathology contributes to clinicopathologic heterogeneity of CBD. Paraffin-embedded sections of the midbrain, pons, subthalamic nucleus, and basal forebrain from 187 autopsy-confirmed CBD cases were screened with immunohistochemistry for phospho-TDP-43. In cases with TDP-43 pathology, additional brain regions (i.e., precentral, cingulate, and superior frontal gyri, hippocampus, medulla, and cerebellum) were immunostained. Hierarchical clustering analysis was performed based on the topographical distribution and severity of TDP-43 pathology, and clinicopathologic and genetic features were compared between the clusters. TDP-43 pathology was observed in 45% of CBD cases, most frequently in midbrain tegmentum (80% of TDP-43-positive cases), followed by subthalamic nucleus (69%). TDP-43-positive CBD was divided into TDP-limited (52%) and TDP-severe (48%) by hierarchical clustering analysis. TDP-severe patients were more likely to have been diagnosed clinically as PSP compared to TDP-limited and TDP-negative patients (80 vs 32 vs 30%, P < 0.001). The presence of downward gaze palsy was the strongest factor for the antemortem diagnosis of PSP, and severe TDP-43 pathology in the midbrain tectum was strongly associated with downward gaze palsy. In addition, tau burden in the olivopontocerebellar system was significantly greater in TDP-positive than TDP-negative CBD. Genetic analyses revealed that MAPT H1/H1 genotype frequency was significantly lower in TDP-severe than in TDP-negative and TDP-limited CBD (65 vs 89 vs 91%, P < 0.001). The homozygous minor allele frequencies in GRN rs5848 and TMEM106B rs3173615 were not significantly different between the three groups. In conclusion, the present study indicates that CBD with severe TDP-43 pathology is a distinct clinicopathologic subtype of CBD, characterized by PSP-like clinical presentations, severe tau pathology in the olivopontocerebellar system, and low frequency of MAPT H1 haplotype.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AGD:
-
Argyrophilic grain disease
- ANOVA:
-
Analysis of variance
- CBD:
-
Corticobasal degeneration
- CBD-OPCA:
-
CBD-olivopontocerebellar atrophy
- CBD-RS:
-
CBD-Richardson syndrome
- CBS:
-
Corticobasal syndrome
- DN:
-
Dystrophic neurite
- FTLD-TDP:
-
Frontotemporal lobar degeneration with TDP-43
- GCI:
-
Glial cytoplasmic inclusion
- NCI:
-
Neuronal cytoplasmic inclusion
- NFT:
-
Neurofibrillary tangle
- PSP:
-
Progressive supranuclear palsy
- TDP-43:
-
Transactive response DNA-binding protein of 43 kDa
References
Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW (2015) Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP type A. Acta Neuropathol 129:53–64. https://doi.org/10.1007/s00401-014-1358-z
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M et al (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503. https://doi.org/10.1212/WNL.0b013e31827f0fd1
Arnold SJ, Dugger BN, Beach TG (2013) TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126:51–57. https://doi.org/10.1007/s00401-013-1110-0
Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8:711–715. https://doi.org/10.1093/hmg/8.4.711
Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54(Suppl 5):S15–S19. https://doi.org/10.1002/ana.10570
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Chen AL, Riley DE, King SA, Joshi AC, Serra A, Liao K, Cohen ML, Otero-Millan J, Martinez-Conde S, Strupp M et al (2010) The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front Neurol 1:147. https://doi.org/10.3389/fneur.2010.00147
Clark RA, Isenberg SJ (2001) The range of ocular movements decreases with aging. J AAPOS 5:26–30 (S1091-8531(01)16106-9)
Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG et al (2000) Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol 47:374–377
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS et al (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. https://doi.org/10.1093/brain/awm305
Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y (2009) Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 117:151–158. https://doi.org/10.1007/s00401-008-0463-2
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R et al (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001. https://doi.org/10.1093/hmg/ddl241
Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM et al (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250. https://doi.org/10.1001/archneurol.2010.254
Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99:663–672
Halliday GM, Hardman CD, Cordato NJ, Hely MA, Morris JG (2000) A role for the substantia nigra pars reticulata in the gaze palsy of progressive supranuclear palsy. Brain 123(Pt 4):724–732
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864. https://doi.org/10.1002/mds.26987
Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A et al (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56:1702–1706
Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Josephs KA (2009) Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord 24:1375–1379. https://doi.org/10.1002/mds.22574
Hyman BT, Trojanowski JQ (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 56:1095–1097
Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS et al (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129:1385–1398. https://doi.org/10.1093/brain/awl078
Josephs KA, Eggers SD, Jack CR Jr, Whitwell JL (2012) Neuroanatomical correlates of the progressive supranuclear palsy corticobasal syndrome hybrid. Eur J Neurol 19:1440–1446. https://doi.org/10.1111/j.1468-1331.2012.03726.x
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450. https://doi.org/10.1007/s00401-013-1211-9
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Koga S, Dickson DW, Bieniek KF (2016) Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 75:963–970. https://doi.org/10.1093/jnen/nlw073
Koga S, Lin W, Walton RL, Ross OA, Dickson DW (2018) TDP-43 pathology in multiple system atrophy: colocalization of TDP-43 and α-synuclein in glial cytoplasmic inclusions. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12485
Koga S, Parks A, Kasanuki K, Sanchez-Contreras M, Baker MC, Josephs KA, Ahlskog JE, Uitti RJ, Graff-Radford N, van Gerpen JA et al (2017) Cognitive impairment in progressive supranuclear palsy is associated with tau burden. Mov Disord 32:1772–1779. https://doi.org/10.1002/mds.27198
Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, Cheshire WP, Wszolek ZK, Rademakers R, Dickson DW (2017) Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 32:246–255. https://doi.org/10.1002/mds.26809
Kokkoroyannis T, Scudder CA, Balaban CD, Highstein SM, Moschovakis AK (1996) Anatomy and physiology of the primate interstitial nucleus of Cajal I. Efferent projections. J Neurophysiol 75:725–739. https://doi.org/10.1152/jn.1996.75.2.725
Konno T, Ross OA, Teive HAG, Slawek J, Dickson DW, Wszolek ZK (2017) DCTN1-related neurodegeneration: perry syndrome and beyond. Parkinsonism Relat Disord 41:14–24 (S1353-8020(17)30211-0)
Kosaka K, Yoshimura M, Ikeda K, Budka H (1984) Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree—a new disease? Clin Neuropathol 3:185–192
Kouri N, Murray ME, Hassan A, Rademakers R, Uitti RJ, Boeve BF, Graff-Radford NR, Wszolek ZK, Litvan I, Josephs KA et al (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275. https://doi.org/10.1093/brain/awr234
Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE, Yen SH, Dickson DW (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752. https://doi.org/10.1007/s00401-013-1087-8
Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NC, Yoon H, Kim J et al (2015) Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 6:7247. https://doi.org/10.1038/ncomms8247
Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW (2011) Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 7:263–272. https://doi.org/10.1038/nrneurol.2011.43
Kovacs GG, Murrell JR, Horvath S, Haraszti L, Majtenyi K, Molnar MJ, Budka H, Ghetti B, Spina S (2009) TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24:1843–1847. https://doi.org/10.1002/mds.22697
Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY et al (2011) Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70:327–340. https://doi.org/10.1002/ana.22424
Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, Paviour DC, Lees AJ (2010) Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133:2045–2057. https://doi.org/10.1093/brain/awq123
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47:1–9
Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, Lai EC, Verny M, Ray-Chaudhuri K, McKee A et al (1997) Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 48:119–125
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. https://doi.org/10.1007/s00401-011-0845-8
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27:472–479. https://doi.org/10.1111/bpa.12424
Mishima T, Koga S, Lin WL, Kasanuki K, Castanedes-Casey M, Wszolek ZK, Oh SJ, Tsuboi Y, Dickson DW (2017) Perry syndrome: a distinctive type of TDP-43 proteinopathy. J Neuropathol Exp Neurol 76:676–682. https://doi.org/10.1093/jnen/nlx049
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, Rawal B, Parisi JE, Petersen RC, Kantarci K et al (2015) Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138:1370–1381. https://doi.org/10.1093/brain/awv050
Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, Pasqualucci CA, Jacob-Filho W, Grinberg LT (2016) higher prevalence of TDP-43 proteinopathy in cognitively normal asians: a clinicopathological study on a multiethnic sample. Brain Pathol 26:177–185. https://doi.org/10.1111/bpa.12296
Nicholson AM, Rademakers R (2016) What we know about TMEM106B in neurodegeneration. Acta Neuropathol 132:639–651. https://doi.org/10.1007/s00401-016-1610-9
Oguro H, Okada K, Suyama N, Yamashita K, Yamaguchi S, Kobayashi S (2004) Decline of vertical gaze and convergence with aging. Gerontology 50:177–181. https://doi.org/10.1159/000076777
Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642. https://doi.org/10.1093/hmg/ddn257
Robinson AC, Thompson JC, Weedon L, Rollinson S, Pickering-Brown S, Snowden JS, Davidson YS, Mann DM (2014) No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease. Neuropathol Appl Neurobiol 40:844–854. https://doi.org/10.1111/nan.12155
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A et al (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. https://doi.org/10.1093/brain/awy146
Rusina R, Kovacs GG, Fiala J, Hort J, Ridzon P, Holmerova I, Strobel T, Matej R (2011) FTLD-TDP with motor neuron disease, visuospatial impairment and a progressive supranuclear palsy-like syndrome: broadening the clinical phenotype of TDP-43 proteinopathies. A report of three cases. BMC Neurol 11:50. https://doi.org/10.1186/1471-2377-11-50
Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR et al (2015) TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 138:3110–3122. https://doi.org/10.1093/brain/awv220
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Trojanowski JQ, Revesz T (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33:615–620. https://doi.org/10.1111/j.1365-2990.2007.00907.x
Tsuboi Y, Josephs KA, Boeve BF, Litvan I, Caselli RJ, Caviness JN, Uitti RJ, Bott AD, Dickson DW (2005) Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord 20:982–988. https://doi.org/10.1002/mds.20478
Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, Saito Y, Arai T, Nishiyama K, Murayama S (2015) Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun 3:35. https://doi.org/10.1186/s40478-015-0215-1
Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564. https://doi.org/10.1097/NEN.0b013e31817713b5
Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M et al (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239. https://doi.org/10.1038/ng.536
Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Dong Y, Hayashi T, Konishi J, Kimura J (1998) Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol 55:609–614
Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S et al (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66. https://doi.org/10.1007/s00401-010-0702-1
Yokoyama JS, Karch CM, Fan CC, Bonham LW, Kouri N, Ross OA, Rademakers R, Kim J, Wang Y, Hoglinger GU et al (2017) Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol 133:825–837. https://doi.org/10.1007/s00401-017-1693-y
Yoshida M (2014) Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 34:555–570. https://doi.org/10.1111/neup.12143
Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA (2015) The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 84:927–934. https://doi.org/10.1212/WNL.0000000000001313
Acknowledgements
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Linda Rousseau, Virginia Phillips, and Ariston L. Librero (Mayo Clinic, Jacksonville) for histologic support, Monica Castanedes-Casey (Mayo Clinic, Jacksonville) for immunohistochemistry support, Laura J. Lewis-Tuffin (Mayo Clinic, Jacksonville) for confocal microscopy support, and Drs. Zbigniew K. Wszolek (Mayo Clinic, Jacksonville), Daniel A. Drubach, and David S. Knopman (Mayo Clinic, Rochester) for contributing patients. This work is supported by NIH Grant P50 NS072187, a Jaye F. and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research, and CBD Solutions Research Grant. DWD and OAR are supported by a NINDS Tau Center without Walls (U54-NS10069). OAR is supported by the R01-NS078086 and the Mayo Clinic Foundation and the Center for Individualized Medicine.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure
1: The thickness of the anterior corpus callosum is measured in a standardized hematoxylin and eosin staining section at the level of the nucleus accumbens. (TIFF 13694 kb)
Supplementary Figure
2: (a) The pontine base is annotated for digital quantification of tau immunoreactivity (CP13). (b) The higher magnification image from a box in (a). (c) The custom-designed color deconvolution algorithm to highlight tau deposits (shown in red). Pretangles and threads are observed and quantified. (TIFF 37455 kb)
Supplementary Figure
3: Double-labeling immunofluorescence (green: phospho-TDP43, red: CD44) shows TDP-43 inclusions in astrocytic processes (arrows) in the superior frontal gyrus. Bar: 20 µm. (TIFF 10075 kb)
Rights and permissions
About this article
Cite this article
Koga, S., Kouri, N., Walton, R.L. et al. Corticobasal degeneration with TDP-43 pathology presenting with progressive supranuclear palsy syndrome: a distinct clinicopathologic subtype. Acta Neuropathol 136, 389–404 (2018). https://doi.org/10.1007/s00401-018-1878-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1878-z