Abstract
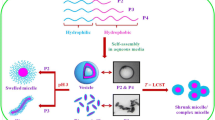
The self-assembly of L-proline functional thermoresponsive double hydrophlic block copolymers (DHBCs) can effectively catalyze aldol reaction in water. Considering the block content of copolymers influences the assemblies and catalytic activities, thermoresponsive DHBCs supported L-proline P(NIPAm-co-ProlA)-b-POEGA with different POEGA block content were prepared by reversible addition fragmentation chain transfer (RAFT) polymerization. The therm-triggered self-assemblies were investigated by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Then, the resultant assemblies were used as catalysts for the aldol reaction with cyclohexanone and 4-nitrobenzaldehyde as substrates. The results indicated that P(NIPAm-co-ProlA)-b-POEGA with ca. 18 mol% of POEGA can form stable catalytic system, which maintained high selectivity and productivity of product, accompanied with satisfactory reusability.







Similar content being viewed by others
References
List B, Lerner RA, Barbas CF (2000) Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc 122:2395–2396
Notz W, List B (2000) Catalytic asymmetric synthesis ofanti-1,2-diols. J Am Chem Soc 122:7386–7387
Gröger H, Wilken J (2001) The application of L-proline as an enzyme mimic and further new asymmetric syntheses using small organic molecules as chiral catalysts. Angew Chem Int Ed 40:529–532
Lindström UM (2002) Stereoselective organic reactions in water. Chem Rev 102:2751–2772
Jarvo ER, Miller SJ (2002) Amino acids and peptides as asymmetric organocatalysts. Tetrahedron 58:2481–2495
Tang Z, Jiang F, Yu LT, Cui X, Gong LZ, Mi AQ (2003) Novel small organic molecules for a highly enantioselective direct aldol reaction. J Am Chem Soc 125:5262–5263
Hayashi Y, Aratake S, Okano T, Takahashi J (2006) Combined proline–surfactant organocatalyst for the highly diastereo- and enantioselective aqueous direct cross-aldol reaction of aldehydes. Angew Chem Int Ed 118:5653–5655
Mukherjee S, Yang JW, Hoffmann S, List B (2007) Asymmetric enamine catalysis. Chem Rev 107:5471–5569
Cordova A, Notz W, Barbas CFIII (2002). Chem Commun 24:3024–3025
Darbre T, Machuqueiro M (2003). Chem Commun 9:1090–1091
Torii H, Nakadai M, Ishihara K, Saito S, Yamamoto H (2004) Asymmetric direct aldol reaction assisted by water and a proline-derived tetrazole catalyst. Angew Chem Int Ed 43:1983–1986
Nyberg AI, Usano A, Pihko PM (2004). Synlett 11:1891–1896
Font D, Sayalero S, Bastero A, Jimeno C, Pericas MA (2008) Toward an artificial aldolase. Org Lett 10:337–340
Mase N, Barbas III CF (2012). Org Biomol Chem 8:4043–4050
Bartok M (2015) Advances in immobilized organocatalysts for the heterogeneous asymmetric direct aldol reactions. Catal Rev 57:192–255
Benaglia M, Puglisi A, Cozzi F (2003) Polymer-supported organic catalysts†. Chem Rev 103:3401–3430
Lu A, Cotanda P, Patterson JP, Longbottom DA, O’Reilly RK (2012) Aldol reactions catalyzed by l-proline functionalized polymeric nanoreactors in water. Chem Commun 48:9699–9701
Huerta E, Stals PJM, Meijer EW, Palmans ARA (2013) Consequences of folding a water-soluble polymer around an organocatalyst. Angew Chem Int Ed 52:2906–2910
Zhang JL, Zhang MX, Tang KJ, Verpoort F, Sun T (2014) Polymer-based stimuli-responsive recyclable catalytic systems for organic synthesis. Small 10:32–46
Upadhyay P, Srivastava V (2016) Proline based organocatalysis: supported and unsupported approach. Curr Organocatal 3:243–269
Lu A, Smart TP, EppsIII TH, Longbottom DA, O’Reilly RK (2011) l-proline functionalized polymers prepared by RAFT polymerization and their assemblies as supported organocatalysts. Macromolecules 44:7233–7241
Klaikherd A, Nagamani C, Thayumanavan S (2009) Multi-stimuli sensitive amphiphilic block copolymer assemblies. J Am Chem Soc 131:4830–4838
Mase N, Nakai Y, Ohara N, Yoda H, Takabe K (2006) Organocatalytic direct asymmetric aldol reactions in water. J Am Chem Soc 128:734–735
Zayas HA, Lu A, Valade D, Amir F, Jia Z, O’Reilly RK, Monteiro MJ (2013) Thermoresponsive polymer-supported l-proline micelle catalysts for the direct asymmetric aldol reaction in water. ACS Macro Lett 2:327–331
Hayashi Y, Sumiya T, Takahashi J, Gotoh H (2006) Highly diastereo- and enantioselective direct aldol reactions in water. Angew Chem Int Ed 45:958–961
Aratake S, Itoh T, Okano T, Nagae N, Sumiya T, Shoji M, Hayashi Y (2007) Highly diastereo- and enantioselective direct aldol reactions of aldehydes and ketones catalyzed by siloxyproline in the presence of water. Chem Eur J 13:10246–10256
Suzuki N, Inoue T, Asada T, Akebi R, Kobayashi G, Rikukawa M, Masuyama Y, Ogasawara M, Takahashi T, Thang SH (2013) Asymmetric aldol reaction on water using an organocatalyst tethered on a thermoresponsive block copolymer. Chem Lett 42:1493–1495
Lu A, Moatsou D, Hands-Portman I, Longbottom DA, O’Reilly RK (2014) Recyclable l-proline functional nanoreactors with temperature-tuned activity based on core–shell nanogels. ACS Macro Lett 3:1235–1239
Boyer C, Bulmus V, Davis TP (2009) Efficient usage of thiocarbonates for both the production and the biofunctionalization of polymers. Macromol Rapid Commun 30:493–497
Frangville C, Li Y, Billotey C, Talham DR, Taleb J, Roux P, Marty J, Mingotaud C (2016) Assembly of double-hydrophilic block copolymers triggered by gadolinium ions: new colloidal MRI contrast agents. Nano Lett 16:4069–4073
Mori H, Kato I, Endo T (2009) Dual-stimuli-responsive block copolymers derived from proline derivatives. Macromolecules 42:4985–4992
An HY, Wang EB, Xiao DR, Li YG, Su ZM, Xu L (2006) Chiral 3D architectures with helical channels constructed from polyoxometalate clusters and copper–amino acid complexes. Angew Chem Int Ed 118:918–922
An Z, Zhang WH, Shi HM, Jing H (2006) An effective heterogeneous l-proline catalyst for the asymmetric aldol reaction using anionic clays as intercalated support. J Catal 241:319–327
Xu J, Ye J, Liu S (2007) Synthesis of well-defined cyclic Poly(N-isopropylacrylamide) via click chemistry and its unique thermal phase transition behavior. Macromolecules 40:9103–9110
Ikegami S, Hamamoto H (2009) Novel recycling system for organic synthesis via designer polymer-gel catalysts. Chem Rev 109:583–593
Jiang X, Zhang G, Narain R, Liu S (2009) Fabrication of two types of shell-cross-linked micelles with “inverted” structures in aqueous solution from schizophrenic water-soluble ABC triblock copolymer via click chemistry. Langmuir 25:2046–2054
Cheng H, Shen L, Wu C (2006) LLS and FTIR studies on the hysteresis in association and dissociation of poly(n-isopropylacrylamide) chains in water. Macromolecules 39:2325–2329
Jiang X, Feng C, Lu GL, Huang XY (2015) Synthesis of temperature and pH/CO2 responsive homopolymer bearing oligo(ethylene glycol) unit and N,N-diethylamino ethyl group and its solution property. Polymer 64:268–276
Yan J, Ji W, Chen E, Li Z, Liang D (2008) Association and aggregation behavior of poly(ethylene oxide)-b-poly (n-isopropylacrylamide) in aqueous solution. Macromolecules 41:4908–4913
Kujawa P, Winnik FM (2001) Volumetric studies of aqueous polymer solutions using pressure perturbation calorimetry: a new look at the temperature-induced phase transition of poly(N-isopropylacrylamide) in water and D2O. Macromolecules 34:4130–4135
Xia Y, Burke NAD, Stöver HDH (2006) End Group effect on the thermal response of narrow-disperse poly(n-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 39:2275–2283
Khimani M, Yusa S, Nagae A, Enomotob R, Aswalc VK, Kesselmand E, Daninod D, Bahadura P (2015) Self-assembly of multi-responsive poly(N-isopropylacrylamide)-b-poly(N,N-dimethylaminopropylacrylamide) in aqueous media. Eur Polym J 69:96–109
Cho EC, Lee J, Cho K (2003) Role of bound water and hydrophobic interaction in phase transition of poly(n-isopropylacrylamide) aqueous solution. Macromolecules 36:9929–9934
Suchao-in N, Chirachanchai S, Perrier S (2009) pH- and thermo-multi-responsive fluorescent micelles from block copolymers via reversible addition fragmentation chain transfer (RAFT) polymerization. Polymer 50:4151–4158
Funding
This work was financially supported by the National Nature Science Foundation of China (Grant No.21403151), the Natural Science Foundation of Shanxi Province (Grant No.201601D011032), the Natural Science Foundation for Young Scientists of Shanxi Province (Grant No. 201601D021045), and the Joint Postgraduate Training base Personnel Training Project of Shanxi Province (Grant No.2017JD17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 1269 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Xu, W., Wang, Q. et al. Self-assembly of L-proline functional thermoresponsive double hydrophlic block copolymers for aldol reaction in water: the influence of POEGA block content. Colloid Polym Sci 296, 1109–1117 (2018). https://doi.org/10.1007/s00396-018-4327-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4327-6