Abstract
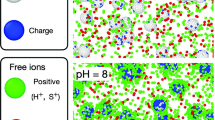
To investigate the effect of hydrophobicity on the charge reversal of colloidal particles, we measured and analyzed the electrophoretic mobility (EPM) of carboxyl latex particles in mixed electrolytes solutions containing potassium chloride KCl and tetraphenylphosphonium chloride TPPCl. Tetraphenylphosphonium (TPP+) ion strongly adsorbs on the particle surface due to its hydrophobicity and thus causes the charge reversal/overcharging. Measurements of EPM were carried out as functions of pH, ionic strength, and the mixed molar ratio of X = [TPP+]/[K+] to unveil the influence of surface charge on hydrophobic interaction. Experimental EPM was analyzed by using 1-pK H Stern Gouy Chapmann model with the Ohshima equation including the relaxation effect or the Smoluchowski equation neglecting the relaxation effect for calculating theoretical EPM values. Our results demonstrate that carboxyl latex particles show charge reversal indicated by positive EPMs at low pH due to the accumulation of TPP+ ions on the surface and the reversed EPM values at low pH are augmented with increasing the mixed molar ratio of X = [TPP+]/[K+]. Also, we observed that charge re-reversal at higher pH as the deprotonation of surface carboxyl groups proceeded. The pH at which the charge re-reversal occurred increased with increasing the mixed molar ratio. From the comparison between our experiments and theoretical analysis, we found that the intrinsic energy of adsorption decreases with increasing the surface charge density to describe the observed charge re-reversal. These results indicate that the intrinsic adsorption energy of TPP+, which is probably due to hydrophobic interaction, decreases with increasing the surface charge density.

Similar content being viewed by others
References
Russel WB, Saville DA, Schowalter WR (1989) Colloidal dispersions, Cambridge University Press
Elimelech M, Gregory J, Jia X (1998) Particle deposition and aggregation: measurement, modelling and simulation, Butterworth-Heinemann
Derjaguin B, Landau L (1941) The theory of stability of highly charged lyophobic sols and coalescence of highly charged particles in electrolyte solutions. Acta Physicochim URSS 14:633–662
Verwey E, Overbeek J, Overbeek J (1999) Theory of the stability of lyophobic colloids, DoverPublications.com
Szilagyi I, Trefalt G, Tiraferri A, Maroni P, Borkovec M (2014) Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 10(15):2479–2502
Tan WF, Norde W, Koopal LK (2011) Humic substance charge determination by titration with a flexible cationic polyelectrolyte. Geochim Cosmochim Acta 75(19):5749–5761
Somasundaran P, Healy TW, Fuerstenau DW (1964) Surfactant adsorption at the solid–liquid interface–dependence of mechanism on chain length. J Phys Chem 68(12):3562–3566
Kobayashi M, Yuki S, Adachi Y (2016) Effect of anionic surfactants on the stability ratio and electrophoretic mobility of colloidal hematite particles. Colloids Surf A Physicochem Eng Asp 510:190–197
Jiménez ML, Delgado ÁV, Lyklema J (2012) Hydrolysis versus ion correlation models in electrokinetic charge inversion: establishing application ranges. Langmuir 28(17):6786–6793
Nishiya M, Sugimoto T, Kobayashi M (2016) Electrophoretic mobility of carboxyl latex particles in the mixed solution of 1:1 and 2:1 electrolytes or 1:1 and 3:1 electrolytes: experiments and modeling. Colloids Surf A Physicochem Eng Asp 504:219–227
Cao T, Sugimoto T, Szilágyi I, Trefalt G, Borkovec M (2017) Heteroaggregation of oppositely charged particles in the presence of multivalent ions. Phys Chem Chem Phys 19:15160–15171
Lyklema J (2006) Overcharging, charge reversal: chemistry or physics? Colloids Surf A Physicochem Eng Asp 291(1–3):3–12
Quesada-Pérez M, González-Tovar E, Martín-Molina A, Lozada-Cassou M, Hidalgo-Álvarez R (2005) Ion size correlations and charge reversal in real colloids. Colloids Surf A Physicochem Eng Asp 267(1–3):24–30
Martín-Molina A, Maroto-Centeno JA, Hidalgo-Álvarez R, Quesada-Pérez M (2008) Charge reversal in real colloids: experiments, theory and simulations. Colloids Surf A Physicochem Eng Asp 319(1–3):103–108
Vereda F, Martín Molina A, Hidalgo-Alvarez R, Quesada-Pérez M (2015) Specific ion effects on the electrokinetic properties of iron oxide nanoparticles: experiments and simulations. Phys Chem Chem Phys 17(17):17069–17078
Calero C, Faraudo J, Bastos-González D (2011) Interaction of monovalent ions with hydrophobic and hydrophilic colloids: charge inversion and ionic specificity. J Am Chem Soc 133(38):15025–15035
Martín-Molina A, Calero C, Faraudo J, Quesada-Pérez M, Travesset A, Hidalgo-Álvarez R (2009) The hydrophobic effect as a driving force for charge inversion in colloids. Soft Matter 5(7):1350
Fuentes LP, Drummond C, Faraudo J, Bastos-González D (2015) Anions makes the difference: insights from the interaction of big cations and anions with poly(N-isopropylacrylamide) chains and microgels. Soft Matter 11:5077
Hakim A, Nishiya M, Kobayashi M (2016) Charge reversal of sulfate latex induced by hydrophobic counterion: effects of surface charge density. Colloid Polym Sci 294(10):1671–1678
Besteman K, Zevenbergen M a G, Lemay SG (2005) Charge inversion by multivalent ions: dependence on dielectric constant and surface-charge density. Phys Rev E Stat Nonlinear Soft Matter Phys 72:1–9
Sipos P, May PM, Hefter GT (2000) Carbonate removal from concentrated hydroxide solutions. Analyst 125(5):955–958
Behrens SH, Christl DI, Emmerzael R, Schurtenberger P, Borkovec M (2000) Charging and aggregation properties of carboxyl latex particles: experiments versus DLVO theory. Langmuir 16(12):2566–2575
Sugimoto T, Kobayashi M, Adachi Y (2014) The effect of double layer repulsion on the rate of turbulent and Brownian aggregation : experimental consideration. Colloids Surf A Physicochem Eng Asp 443:418–424
Schudel M, Behrens SH, Holthoff H, Kretzschmar R, Borkovec M (1997) Absolute aggregation rate constants of hematite particles in aqueous suspensions: a comparison of two different surface morphologies. J Colloid Interface Sci 196(2):241–253
Ohshima H, Healy TW, White LR (1983) Approximate analytic expressions for the electrophoretic mobility of spherical colloidal particles and the conductivity of their dilute suspensions. J Chem Soc Faraday Trans 2 79(11):1613
The Chemical Society of Japan (2004) Ed., Kagaku Binran (ed), Maruzen, in Japanese
Zhao Y, Freeman GR (1996) Electrical conductances of tetraphenylphosphonium and tetraphenylboride salts in C 1 to C 4. Alcohols 3654(96):17568–17572
Ehrl L, Jia Z, Wu H, Lattuada M, Soos M, Morbidelli M (2009) Role of counterion association in colloidal stability. Langmuir 25(13):2696–2702
Kim JI (1978) Preferential solvation of single ions. A critical study of the Ph4AsPh4B assumption for single ion thermodynamics in amphiprotic and dipolar-aprotic solvents. J Phys Chem 82(2):191–199
Funding
This work was supported by JSPS KAKENHI Grant Number 15H04563, 16H06382, and 15J00805.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sugimoto, T., Nishiya, M. & Kobayashi, M. Electrophoretic mobility of carboxyl latex particles: effects of hydrophobic monovalent counter-ions. Colloid Polym Sci 295, 2405–2411 (2017). https://doi.org/10.1007/s00396-017-4219-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4219-1